- *Corresponding Author:
- Y. Cui
Department of Nephrology, The First Peoples’ Hospital of Chenzhou, Chenzhou 423000, Hunan Province, China
E-mail: sanlongtree@126.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “98-104” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study summarizes the efficacy of roxadustat for secondary hyperparathyroidism. 65 individuals with secondary hyperparathyroidism were selected between May 2022 and May 2023 and were divided into control (n=30) and observation groups (n=35). The control group was treated with iron polysaccharide complex capsules+erythropoietin while the observation group was given roxadustat. Comparative analysis was conducted in terms of efficacy, drug safety which included fatigue, nausea and vomiting, myodynia and hypercalcemia. Further, serum biochemical indices like red blood cell count, hematocrit value and hemoglobin concentration were observed and compared. Then, microinflammation indicators like C-reactive protein, beta 2-microglobulin and interleukin-6 were also evaluated. Finally, the quality of life according to the World Health Organization quality of life was evaluated. The results revealed markedly higher efficacy, and a comparable incidence of adverse drug events in the observation group compared with the control group. In addition, the observation group had evidently elevated red blood cell count, hematocrit value, hemoglobin and World Health Organization quality of life scores compared with the baseline and the control group, as well as significantly higher reduced C-reactive protein, beta 2-microglobulin and interleukin-6. It can be concluded that, roxadustat is effective and safe for secondary hyperparathyroidism patients which can ameliorate anemia-related indicators and the microinflammation indicators can enhance the quality of life.
Keywords
Hyperparathyroidism, roxadustat, erythropoietin, chronic kidney disease
Secondary Hyperparathyroidism (SHPT) is one of the common complications of kidney disease, and its main pathological manifestations are parathyroid hyperplasia, excessive secretion of intact Parathyroid Hormone (iPTH), and disturbance of Calcium (Ca) and Phosphate (P) metabolism[1-3]. The disease can cause ectopic calcification, myasthenia, skin itching, bone and joint pain and other clinical symptoms, which can be even life-threatening[4,5]. Current treatment options for the disease include vitamin D therapy, calcimimetic agents, P binders, etc. However, vitamin D therapy has the limitation of narrow treatment window, calcimimetic agents may lead to unbearable side effects and high medical expenses, and P binders have a limited range of application[6-8]. Therefore, we believe that it is necessary to explore more effective treatment strategies for SHPT to improve the efficacy of SHPT patients and alleviate clinical symptoms.
Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor that promotes erythropoiesis and participates in the regulation of iron metabolism, is as effective in anaemia in dialysis patients as the standard therapy epoetin Alpha (α)[9]. Besides, the drug can be used to treat anemia in patients with dialysis-/non-dialysis-dependent Chronic Kidney Disease (CKD) and myelodysplastic syndrome[10]. A rat CKD model experiment shows that roxadustat validly inhibited the excessive increase of iPTH and Fibroblast Growth Factor 23 (FGF23) in CKD rats, while alleviating bone loss and bone microstructure deterioration by regulating osteoblast activity and osteoclast activity, suggesting its potential therapeutic value in SHPT[11]. In addition, traditional iron and Erythropoietin (EPO) therapy is less effective in SHPT patients with refractory anemia. Roxadustat, on the other hand, can produce endogenous EPO without iron supplementation while ameliorating microinflammatory status, which is more effective in such patients[12-14].
Given the paucity of studies on the clinical use of roxadustat in the treatment of SHPT, this study sought to compare it with iron polysaccharide complex capsules+EPO to validate its potential therapeutic advantages in SHPT.
Materials and Methods
General information:
In this study, 65 SHPT individuals who have been admitted to our hospital between May 2022 and May 2023 were strictly screened out according to the inclusion and exclusion criteria. Among them, 30 individuals assigned in the control group were treated with iron polysaccharide complex capsules+EPO while 35 individuals assigned as observation group were treated with roxadustat alone. The clinical comparability was identified between groups with no statistical differences in general information (p>0.05). This study was approved by the ethics committee approval from our hospital.
Inclusion criteria:
All the patients who met the diagnostic criteria for SHPT, i.e., Parathyroid Hormone (PTH) level with 300-800 pg/ml and Hemoglobin (Hb) level having 60-110 mmHg; patients with the indications for hemodialysis; patients with SHPT developed during dialysis; patients with uninterrupted hemodialysis >3 mo before enrollment and patients having no history of drug allergy, and those having complete clinical data were included in this study.
Exclusion criteria:
Patients having prior history of parathyroid diseases; patients have used immunosuppressants in the past 1 mo; patients having the history of blood transfusion within the last mo; patients who intend to undergo kidney transplantation or parathyroidectomy and those suffering from hematological diseases or autoimmune system deficiencies were excluded from the study.
Treatment method:
The control group was treated with iron polysaccharide complex capsules+EPO. Patients were given 150 mg of iron polysaccharide complex capsules orally once a day, along with subcutaneous injection of 10 000 units of EPO once a week. The frequency of medication did not exceed once every 2 d, and the dose was adjusted weekly according to the patient's iPTH level. Similarly, the observation group was treated with 100 mg/time roxadustat, three times a week for 12 w.
Endpoints:
Efficacy: The recovery of clinical symptoms and biochemical indices before and after treatment was used as the efficacy evaluation criteria. This criterion included, markedly effective, effective and ineffective. Markedly effective corresponds if the symptoms basically disappeared, if the levels of iPTH, blood Ca and blood P returned to normal. Similarly, effective means that the symptoms are found to be improved, the levels of iPTH, blood Ca and blood P recovered significantly; while ineffective refers if the above indicators did not change, and the condition even worsened.
Total effective rate=Sum of the number of markedly effective+effective patients/the total number of cases (n)×100
Safety: We mainly evaluated by observing and recording the number of adverse events such as fatigue, nausea and vomiting, myodynia and hypercalcemia in the two groups.
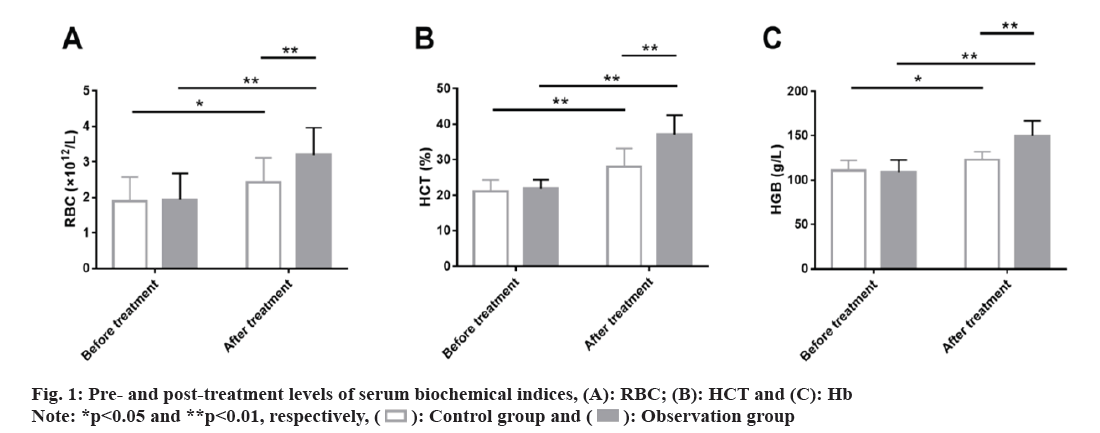
Serum biochemical indices: 5 ml of fasting cubital venous blood was collected from each patient before and after treatment. The levels of Red Blood Cell (RBC) count, Hematocrit (HCT) and Hb concentration were determined using an automatic biochemical analyzer.
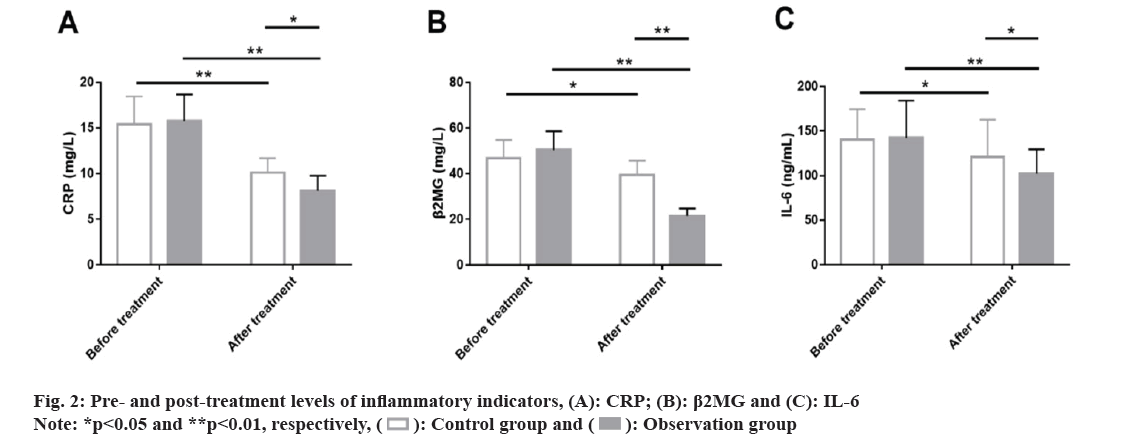
Inflammatory indicators: The pre- and post-treatment levels of C-reactive protein (CRP), Beta 2-Microglobulin (β2MG) and Interleukin (IL)-6 of the patients in both the groups were measured using the Enzyme Linked Immunosorbent Assay (ELISA).
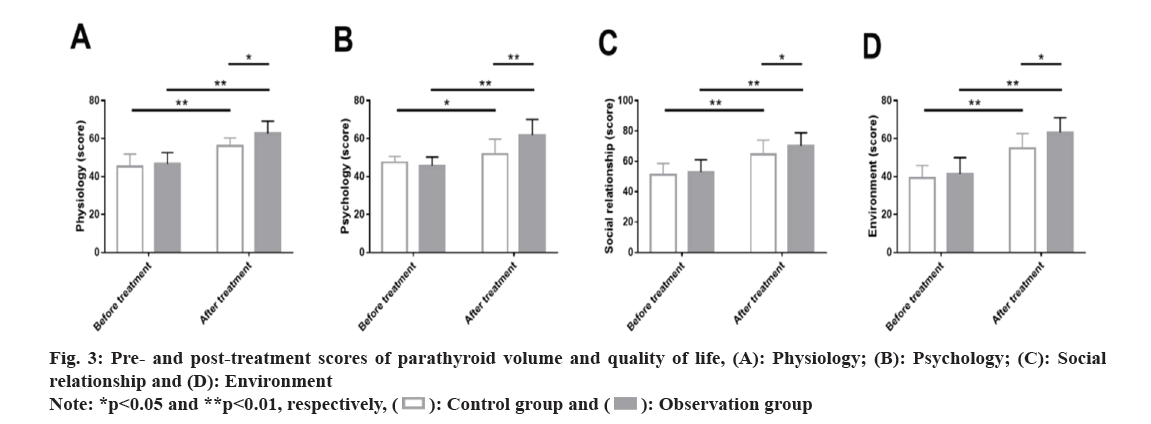
Quality of life: Changes in patients’ quality of life were evaluated by the World Health Organization Quality of Life Brief Version (WHOQOL-BREF) from physiology, psychology, social relationship, and environment domains, with the score ranging from 0 to 100 points; higher scores indicate better quality of life.
Statistical analysis:
In this study, continuous variables were measured in terms of mean±Standard Error of Mean (SEM). Inter- and intra-group comparisons were performed using the independent samples t-test and paired t-test, respectively. Categorical variables, expressed by the rate (percentage), were comparatively analyzed with the Chi-square (χ²) test. All the data was analyzed using Statistical Package for Social Sciences (SPSS) version 22.0, with statistical significance reported as p<0.05.
Results and Discussion
No significant inter-group difference was found in gender, age, dialysis duration and other general information between the patients of both the groups (p>0.05) (Table 1).
| Indicators | Control group (n=30) | Observation group (n=35) | χ²/t | p |
|---|---|---|---|---|
| Gender | 0.095 | 0.758 | ||
| Male | 16 (53.33) | 20 (57.14) | ||
| Female | 14 (46.67) | 15 (42.86) | ||
| Age (y) | 57.33±6.42 | 56.77±7.19 | ||
| Dialysis duration (y) | 6.70±2.68 | 6.91±2.73 | ||
| Body Mass Index (BMI) (kg/m2) | 22.21±2.80 | 21.53±2.45 | ||
| Disease type | 1.142 | 0.888 | ||
| Chronic glomerulonephritis | 11 (36.67) | 15 (42.86) | ||
| Diabetic nephropathy | 8 (26.67) | 6 (17.14) | ||
| Hypertensive renal injury | 6 (20.00) | 8 (22.86) | ||
| Obstructive nephropathy | 4 (13.33) | 4 (11.43) | ||
| Polycystic kidney | 1 (3.33) | 2 (5.71) |
Table 1: General information of patients.
The total efficacy between the two groups was evaluated. The total effective rates of the control and observation groups were 70.00 % and 91.43 %, respectively, indicating higher therapeutic effects of roxadustat used in the observation group compared with iron polysaccharide complex capsules+EPO used in the control group (p<0.05) (Table 2).
| Drug effectiveness | Control group (n=30) | Observation group (n=35) | χ² | p |
|---|---|---|---|---|
| Markedly effective | 13 (43.33) | 17 (48.57) | ||
| Effective | 8 (26.67) | 15 (37.14) | ||
| Ineffective | 9 (30.00) | 3 (8.57) | ||
| Total effectiveness | 21 (70.00) | 32 (91.43) | 4.928 | 0.026 |
Table 2: Comparison of therapeutic effects.
The clinical safety was also evaluated. The control and observation groups were not statistically different in the total incidence of fatigue, nausea and vomiting, myodynia and hypercalcemia (16.67 % vs. 11.43 %, p>0.05) (Table 3).
| Indicators | Control group (n=30) | Observation group (n=35) | χ² | p |
|---|---|---|---|---|
| Fatigue | 1 (3.33) | 1 (2.86) | ||
| Nausea and vomiting | 2 (6.67) | 1 (2.86) | ||
| Myodynia | 1 (3.33) | 2 (5.71) | ||
| Hypercalcemia | 1 (3.33) | 0 (0.00) | ||
| Total | 5 (16.67) | 4 (11.43) | 0.372 | 0.542 |
Table 3: Comparison of medication safety.
Further, serum biochemical indices between the two groups were evaluated. The two groups did not differ much in pre-treatment RBC, HCT and HGB (p>0.05). A marked rise in these indices were observed in both groups, with even higher RBC, HCT and HGB in the observation group (p<0.05) (fig. 1).
Similarly, inflammatory indicators such as levels of CRP, β2MG and IL-6 were detected using ELISA in the two groups to evaluate the effects of the two medication methods on the microinflammatory status of patients. The data showed no marked inter-group differences in various microinflammation indicators before treatment (p>0.05); CRP, β2MG and IL-6 were all significantly inhibited in both groups after treatment (p<0.05) with even lower levels of them in the observation group (p<0.05) (fig. 2).
Subsequently, the quality of life of patients after undergoing treatment was evaluated and compared. Similarly, the pre-treatment WHOQOL-BREF were not statistically different between groups in physiology, psychology, social relationship and environment domains (p>0.05). After the intervention, scores of all the dimensions of the WHOQOL-BREF scale increased significantly, with higher scores in the observation group compared with the control group (p<0.05) (fig. 3).
SHPT, which occurs frequently in patients with CKD and maintenance hemodialysis, is the most common and serious adverse event[15]. Our study confirms the significant clinical advantage of roxadustat over iron polysaccharide complex capsules+EPO in the treatment of SHPT and is hereby reported.
According to efficacy evaluation, the total effective rate was higher in the observation group compared with the control group (91.43 % vs. 70.00 %), suggesting that roxadustat is a good treatment for SHPT patients with better efficacy than iron polysaccharide complex capsules+EPO. Roxadustat is reported to produce endogenous EPO and improve the sensitivity of EPO receptors to inhibit hepcidin levels, which enhances iron homeostasis in CKD patients[16]. This helps to inhibit FGF23 levels, which in turn mediates the pathophysiological cascade of SHPT that may be triggered by abnormally high levels of FGF23 and plays a role in the disease inhibition during the pathological development of SHPT[17,18].
In terms of safety, the total incidence of fatigue, nausea and vomiting, myodynia, and hypercalcemia was lower in the observation group than in the control group (11.43 % vs. 16.67 %), indicating that roxadustat treatment can reduce the risk of side effects in SHPT patients by 5.24 %. Roxadustat has also been shown to have a good safety profile in the treatment of renal anemia and a low risk of cardiovascular adverse events in patients with dialysis-dependent CKD anemia[19,20]. In addition, by causing EPO resistance and accumulating PTH, SHPT may shorten red blood cell survival and cause myofibrosis and anemia, resulting in poor prognosis of patients[21-23]. After evaluating the serum biochemical indices related to anemia, it was found that RBC, HCT and HGB of the observation group were significantly improved after treatment, and were significantly higher than those of the control group, suggesting that roxadustat is more effective than iron polysaccharide complex capsules+EPO in alleviating anemia, similar to the findings of Provenzano et al.[24].
The therapeutic mechanism of Roxadustat in SHPT-related anemia may be related to its regulation of Hypoxia-Inducible Factor 1 (HIF)-α, which in turn promotes a range of erythropoietic responses[25]. Fishbane et al.[26] also pointed out that Roxadustat in patients with dialysis-dependent CKD can significantly promote the increase of Hb levels, consistent with our findings. Patients with SHPT are often accompanied by an excessive inflammatory response, and inflammation is often an unconventional risk factor for cardiovascular adverse events and mortality in end-stage kidney disease[27,28]. Therefore, evaluation of microinflammatory status in SHPT patients is essential. In this study, CRP, β2MG and IL-6 in the observation group reduced markedly after treatment, lower compared with the control group, indicating more potent inhibition of inflammation by roxadustat compared with iron polysaccharide complex capsules+EPO. CRP is also shown to be an indicator strongly correlated with anemia in renal cell carcinoma and IL-6, as a strong inducer of CRP and a regulator of iron transport, is also closely related to the prognosis of anemia patients[29]. The WHOQOL-BREF scores assessed from physiology, psychology, social relationship and environment domains showed that the scores of various domains were obviously elevated in the observation group after intervention, higher than those in the control group, indicating that roxadustat intervention has a significant positive effect on the improvement of quality of life of SHPT patients.
In summary, roxadustat has higher efficacy than iron polysaccharide complex capsules+EPO in SHPT patients with a favorable safety profile, which can effectively promote symptom resolution, improve Ca and P metabolism, reduce Post-traumatic Growth (PTG) volume and enhance the quality of life, resulting to provide a new treatment for SHPT patients.
Author’s contribution:
Qiong Wu and Yi Cui designed the research and wrote the manuscript. Qin Zou and Chen-ting Li contributed to conceive the research and analyzed the data. Xiang-ping Liao conducted the analysis and provided guidance for the research and all the authors were involved in reviewing and approved the final manuscript.
Funding:
This study was supported by the 2019 school-level scientific research project of Xiangnan College (Project no: 2019XJ82).
Conflict of interest:
The author declared no conflict of interests.
References
- Yao P, Lei T, Li QM. Efficacy of lanthanum carbonate combined with cinacalcet and paricalcitol in the treatment of hyperparathyroidism secondary to hemodialysis and its effect on iPTH. Chin J Clin Ration Drug Use 2024;9:109-12.
- Zhou CF, Zeng XQ, Zeng QY. Effect of cinacalcet combined with calcitriol on calcium and phosphorus metabolism, serum level of whole parathyroid hormone in patients with secondary hyper-parathyroidism. Northwest Pharm J 2020;35(3):422-5.
- Mizobuchi M, Ogata H, Koiwa F. Secondary hyperparathyroidism: Pathogenesis and latest treatment. Ther Apher Dial 2019;23(4):309-18.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Kang HB, Chen J. Re-operation after parathyroidectomy for the recurrence of the secondary hyperparathyroidism, clinical analysis of 16 cases. Chin J Oper Procedures Gen Surg 2020;14(6):1-4.
- Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: A review. JAMA 2019;322(13):1294-304.
[Crossref] [Google Scholar] [PubMed]
- Lau WL, Obi Y, Kalantar ZK. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol 2018;13(6):952-61.
[Crossref] [Google Scholar] [PubMed]
- Yang ZW. Comparison of short-term efficacy of surgical treatment and oral cinacalcet therapy for refractory secondary hyperparathyroidism. Xinjiang Med Univ 2010;11:21-5.
- Feng XW. Clinical efficacy of active vitamin D and phosphate binder combined with cinacalcet hydrochloride in the treatment of secondary hyperparathyroidism in dialysis patients with chronic renal failure. Chin J Clin Ration Drug Use 2016;9(6):91-2.
- Ren X, Guo YB, Zhang LY. Effect of roxadustat on blood biochemistry,iron metabolism and calcium-phosphorus metabolism in patients undergoing hemodialysis for renal anemia inadequately controlled by rHuEPO. Shaanxi Med J 2023;52(7):902-5.
- Lu XF, Zhang YN. Observation on the efficacy and changes in iron metabolism of standard initial dose roxadustat in the treatment of non-dialysis renal anemia patients with different CKD stages. Chin J Pract Intern Med 2024;2:92-7.
- Li L, Li A, Gan L, Zuo L. Roxadustat improves renal osteodystrophy by dual regulation of bone remodeling. Endocrine 2023;79(1):180-9.
[Crossref] [Google Scholar] [PubMed]
- Schaefer B, Meindl E, Wagner S, Tilg H, Zoller H. Intravenous iron supplementation therapy. Mol Aspects Med 2020;75:1-12.
[Crossref] [Google Scholar] [PubMed]
- Weir MR. Managing anemia across the stages of kidney disease in those hyporesponsive to erythropoiesis-stimulating agents. Am J Nephrol 2021;52(6):450-66.
[Crossref] [Google Scholar] [PubMed]
- Duan CY, He YM, Huang QE. Effect mechanism of roxastat in the treatment of maintenance hemodialysis complicated with anemia based on PHD2/HIF-2a/EPO pathway. Cap Med 2021;28(18):63-5.
- Meola M, Petrucci I, Cupisti A. Ultrasound in clinical setting of secondary hyperparathyroidism. J Nephrol 2013;26(5):848-55.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Liu CY, Xu LY. Efficacy of roxadustat in the treatment of renal anemia in peritoneal dialysis patients with poor EPO efficacy. Chin J Integr Tradit West Nephrol 2022;23(4):365-7.
- Zhao WW, Wang YF, Wang MC. Effect of roxadustat on anemia and FGF-23 of patients with maintenance hemodia lysis. Pract Pharm Clin Remedies 2023;11:1022-6.
- Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 2018;3(23):1-12.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Jiang L, Wei X, Long M, Du Y. Roxadustat: Not just for anemia. Front Pharmacol 2022;13:1-13.
[Crossref] [Google Scholar] [PubMed]
- Sun YL, Xie H, Kang Z, Zhang SK, Zhao TT, Wang BW. Roxadustat treatment for anemia in 20 cases of patients undergoing initial-stage hemodialysis. Chin J Pract Int Med 2020;40(11):1-5.
- Tanaka M, Yoshida K, Fukuma S, Ito K, Matsushita K, Fukagawa M, et al. Effects of secondary hyperparathyroidism treatment on improvement in anemia: Results from the MBD-5D study. PLoS One 2016;11(10):1-13.
[Crossref] [Google Scholar] [PubMed]
- Villain C, Ecochard R, Bouchet JL, Daugas E, Drueke TB, Hannedouche T, et al. Relative prognostic impact of nutrition, anaemia, bone metabolism and cardiovascular comorbidities in elderly haemodialysis patients. Nephrol Dial Transplant 2019;34(5):848-58.
[Crossref] [Google Scholar] [PubMed]
- Rroji M, Spasovski G. Calcimimetics vs. parathyroidectomy: What is preferable? Int Urol Nephrol 2018;50(7):1271-5.
[Crossref] [Google Scholar] [PubMed]
- Provenzano R, Shutov E, Eremeeva L, Korneyeva S, Poole L, Saha G, et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant 2021;36(9):1717-30.
[Crossref] [Google Scholar] [PubMed]
- Liu BH, Wang MZ, Sun W. Molecular regulatory mechanisms of hypoxia-inducible factor and interventional effect of Chinese herbal medicine. China J Chin Mater Med 2020;45(20):1-7.
- Fishbane S, Pollock CA, El-Shahawy M, Escudero ET, Rastogi A, van BP, et al. Roxadustat vs. epoetin alpha for treating anemia in patients with chronic kidney disease on dialysis: Results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol 2022;33(4):850-66.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Tan YY, Zhou JJ. Efficacy of paricalcitol combined with ciracalcet on the secondary hyperparathyroidisim and micro-inflammatory state for patients undergoing hemodialysis. J Changzhi Med Coll 2023;37(5):336-9.
- Nasif WA, Mukhtar MH, El-Emshaty HM, Alwazna AH. Redox state of human serum albumin and inflammatory biomarkers in hemodialysis patients with secondary hyperparathyroidism during oral calcitriol supplementation for vitamin D. Open Med Chem J 2018;12:98-110.
[Crossref] [Google Scholar] [PubMed]
- Falkensammer CE, Thurnher M, Leonhartsberger N, Ramoner R. C-reactive protein is a strong predictor for anaemia in renal cell carcinoma: Role of IL-6 in overall survival. BJU Int 2011;107(12):1893-8.
[Crossref] [Google Scholar] [PubMed]
 .
.