- *Corresponding Author:
- Huiping Li
Department of Nursing, Anhui Medical University, Anhui Province 230032, China
E-mail: MAGI-F@outlook.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “192-198” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study seeks to elucidate the efficacy of oxaliplatin, fluorouracil and calcium folinate combined with hepatic arterial infusion chemotherapy, in patients with advanced primary liver cancer. In this study, 156 advanced primary liver cancer individuals who were treated in our hospital between July 2018 and July 2021 were selected among which 70 patients who received folinic acid/calcium folinate, fluorouracil and oxaliplatin regimen via conventional intravenous infusion were assigned as control group and 86 patients who received hepatic arterial infusion chemotherapy using the folinic acid/calcium folinate, fluorouracil and oxaliplatin regimen were included in the research group. The two groups were compared with respect to their curative effects, complication rate (nausea and vomiting, fatigue, fever, abnormal liver function and thrombocytopenia) and improvement of health status using Karnofsky performance scores. Similarly serum indices such as vascular endothelial growth factor, alpha-fetoprotein, cancer antigen 19-9 and 1 y overall survival were also evaluated. Significantly higher overall effectiveness, better improvement of health status (high Karnofsky performance scores) and fewer complications were found in the research group when compared with the control group; the post-treatment vascular endothelial growth factor, alphafetoprotein, cancer antigen 19-9 of the research group reduced statistically and were lower compared with the control group. Notably higher 1 y overall survival was determined in the research group. Therefore, hepatic arterial infusion chemotherapy using the folinic acid/calcium folinate, fluorouracil and oxaliplatin regimen is beneficial to improve the clinical outcomes of advanced primary liver cancer patients with a good preventive effect on the occurrence of complications, which can not only significantly improve patients’ health status, inhibit vascular endothelial growth factor, alpha-fetoprotein, cancer antigen 19-9 levels, but also significantly increase the 1 y overall survival of patients.
Keywords
Oxaliplatin, fluorouracil, calcium folinate, hepatic arterial infusion chemotherapy, advanced primary liver cancer
Primary Liver Cancer (PLC), one of the common fatal tumors in the world, is etiologically closely linked to alcoholism, obesity, metabolic syndrome, type 2 diabetes and nonalcoholic fatty liver disease besides Hepatitis B Virus (HBV) and HCV infections[1]. According to the relevant epidemiological data, Hepatocellular Carcinoma (HCC) is the major pathological type of PLC, accounting for up to 90 % of the total cases. The global mortality rate of HCC is about 8.5 out of 100 000 people and the incidence is also rising highly[2,3]. Early HCC is primarily treated by radical treatment such as surgical resection, local ablation and liver transplantation, which contributes to a median survival exceeding 5 y[4,5]. However, the median survival time will drop to about 2 y in PLC cases diagnosed in the middle and late stages, when the above treatment options are not suitable[6]. Although the Folinic acid/calcium folinate, Fluorouracil and Oxaliplatin (FOLFOX) chemotherapy regimen can be used to treat advanced HCC, conventional intravenous infusion may limit therapeutic effectiveness due to the low concentration of the drug at the tumor site[7]. In addition, the high side effects associated with this therapy may also make it difficult for patients to tolerate[8]. Therefore, this study sought to explore a better treatment strategy for patients with advanced HCC based on FOLFOX chemotherapy regimen to improve the clinical outcome of patients.
The FOLFOX regimen is composed of Oxaliplatin (OXA), Fluorouracil (FU), and Calcium Folinate (CF), among which OXA, as a Deoxyribonucleic Acid (DNA) synthesis inhibitor superior to cisplatin, is also effective in the treatment of HCC patients with a diameter >10 cm when used in continuous Hepatic Arterial Infusion Chemotherapy (HAIC)[9]. It can also play a synergistic role with FU and CF in the treatment of HCC, which helps to improve the survival outcome of advanced HCC patients to a certain extent[10]. FOLFOX regimen has been shown to be more effective and safe in patients with large unresectable HCC and is superior to Transcatheter Arterial Chemoembolization (TACE) alone[11]. In addition, modified FOLFOX regimen with HAIC as an implementation mode can be used as an alternative treatment strategy for advanced HCC when TACE is ineffective or inappropriate[12]. At present, the research on the clinical advantages of FOLFOXHAIC regimen for advanced PLC is still limited.
This study attempts to analyze the influence of FOLFOX (OXA+FU+CF)-HAIC regimen on clinical outcomes of advanced PLC patients, in order to provide new clues for the treatment and prognosis improvement of such patients.
Materials and Methods
The participants were 156 advanced PLC patients from July 2018 to July 2021. Among them, 86 patients in the research group received FOLFOXHAIC, and 70 patients in the control group received FOLFOX via routine intravenous infusion. The gender parity and mean age (y) of research group were 53:33 and (56.97±9.25), respectively; control group was composed of 45 males and 25 females aged (59.03±8.14) y on an average. Research and control groups were clinically comparable with no evident difference in gender, age and other baseline data. The Ethics Committee of the Third Affiliated Hospital of Anhui Medical University (No: 202107) has approved for this research study.
Inclusion criteria:
Patients diagnosed with advanced PLC[13]; patients having no contraindications to the treatment methods in this study; patients who had completed 1 y follow-up; patients whose estimated survival is ≥3 mo; patients who did not undergo any treatment intervention in recent 1 mo were included in this study.
Exclusion criteria:
Patients with other liver diseases, other malignant tumors or serious organ diseases; patients who underwent other treatments; pregnant or lactating women; patients with mental illness; patients incomplete clinical medical records were excluded from this study.
Methods:
Patients in both research and control groups received routine care and basic treatment.
Primarily the patients in control group received 85 mg/m2 of OXA on the 1st d, 200 mg/m2 of CF, 400 mg/m2 of FU and 600 mg/m2 of FU on the 1st and 2nd d. Each chemotherapy session lasted for 2 w.
Similarly patients in research group underwent hepatic artery catheterization in the Digital Subtraction Angiography (DSA) catheterization room of our hospital and femoral artery puncture using the Seldinger technique. After celiac trunk artery or hepatic artery angiography, the microcatheter was inserted superselectively to the tumor feeding artery as far as possible and the puncture point of the femoral artery was fixed externally. After the patient returned to the wards, the catheter was connected to an arterial infusion pump and received an arterial infusion of OXA (130 mg/m2) for 1.5 h, followed by arterial infusion of CF (200 mg/m2) for 1.5 h; bolus infusion of 400 mg/m2 of FU was also given on the 1st d, followed by arterial infusion of 2400 mg/ m2 of FU for 46 h. After the treatment, the arterial indwelling catheter was removed and the puncture site was pressed to stop bleeding. Postoperative fluid replacement, support and symptomatic treatment were given. Patients in research group received HAIC treatment, with a cumulative treatment of 36 times.
Detection indicators:
Efficacy: Complete Response (CR) corresponds to the complete disappearance of all measurable lesions and the absence of new lesions, lasting ≥4 w. Partial Response (PR) is defined as a >50 % reduction in the total maximum length diameter of all measurable lesions, lasting for ≥4 w. Stable Disease (SD) corresponds to reduction of the issue to <50 % or a <25 % increase in the sum of the all measurable lesions (length in diameters). Progressive Disease (PD) is defined as a >25 % increase in the total maximum length and diameter of the tumor or the appearance of new lesions.
Complication rate: We observed and counted the cases of nausea, vomiting, fatigue, fever, abnormal liver function and thrombocytopenia in the two groups and calculated the total incidence.
Improvement of health status: The improvement of patients’ health status was assessed using the Karnofsky Performance Status (KPS) score; range having 0-100[14], with a post-treatment KPS score increased by >10 points, altered by ≤10 points and decreased by >10 points indicating improved, stabilized and deteriorated, respectively.
Serum indices: 5 ml of fasting cubital venous blood was collected before and after treatment and was centrifuged to isolate serum samples. Enzyme- Linked Immunosorbent Assay (ELISA) was used to determine Vascular Endothelial Growth Factor (VEGF), Alpha-Fetoprotein (AFP) and Cancer Antigen 19-9 (CA19-9). The procedure strictly followed the kit instructions.
1 y Overall Survival (OS): Patients were followed up for 1 y by means of medical records, telephone visits and outpatient visits, etc. For every 3 mo, all the patients were followed up and the 1 y OS was recorded.
Statistical analysis:
The number of cases/percentage (n/%) and the mean±Standard Error of Mean (mean±SEM) are used to represent categorical and continuous variables, respectively. Paired t-test and independent sample t-test were respectively used for intra- and inter-group comparison before and after treatment.
The statistical analysis, relied upon a p<0.05 using Graphpad Prism version 7.0 software.
Results and Discussion
Research group and control group showed no marked differences in sex, age, Child-Pugh liver function classification, tumor stage, AFP and tumor diameter (p>0.05), showing clinical comparability (Table 1).
| Indicators | Research group (n=86) | Control group (n=70) | χ2/t | p |
|---|---|---|---|---|
| Gender | 0.117 | 0.733 | ||
| Male | 53 (61.63) | 45 (64.29) | ||
| Female | 33 (38.37) | 25 (35.71) | ||
| Age (y) | 56.97±9.25 | 59.03±8.14 | 1.459 | 0.147 |
| Child-Pugh liver function classification | 0.864 | 0.353 | ||
| A | 62 (72.09) | 55 (78.57) | ||
| B | 24 (27.91) | 15 (21.43) | ||
| Tumor stage | 0.145 | 0.703 | ||
| III | 49 (56.98) | 42 (60.00) | ||
| IV | 37 (43.02) | 28 (40.00) | ||
| AFP (μg/l) | 0.361 | 0.548 | ||
| <400 | 45 (52.33) | 40 (57.14) | ||
| ≥400 | 41 (47.67) | 30 (42.86) | ||
| Tumor diameter (cm) | 2.231 | 0.135 | ||
| <5 | 68 (79.07) | 48 (68.57) | ||
| ≥5 | 18 (20.93) | 22 (31.43) |
Table 1: Baseline Information of the Patients
The total effective rate of the research group was 70.93 %, which was significantly >42.86 % compared with the control group (p<0.05) (Table 2).
| Indicators | Research group (n=86) | Control group (n=70) | χ2/t | p |
|---|---|---|---|---|
| CR | 3 (3.49) | 0 (0.00) | ||
| PR | 58 (67.44) | 30 (42.86) | ||
| SD | 20 (23.26) | 22 (31.43) | ||
| PD | 5 (5.81) | 18 (25.71) | ||
| Total effective rate | 61 (70.93) | 30 (42.86) | 12.510 | <0.001 |
Table 2: Efficacy of the Two Groups
Complication rate of the two groups was studied. The number of cases with nausea, vomiting, fatigue, fever, abnormal liver function and thrombocytopenia in the two groups were observed and counted to calculate the incidence of corresponding complications. The statistical results identified dramatically lower incidence rates of the above complications in research group vs. control group (p<0.05) (Table 3).
| Indicators | Research group (n=86) | Control group (n=70) | χ2/t | p |
|---|---|---|---|---|
| Nausea and vomiting | 15 (17.44) | 31 (44.29) | 13.370 | 3.657 |
| Fatigue | 9 (10.47) | 16 (22.86) | 4.403 | 0.036 |
| Fever | 14 (16.28) | 22 (31.43) | 4.989 | 0.026 |
| Abnormal liver function | 5 (5.81) | 14 (20.00) | 7.261 | 0.007 |
| Thrombocytopenia | 20 (23.26) | 29 (41.43) | 5.915 | 0.015 |
Table 3: Incidence of Complications Rate in Two Groups
Improvement of health status in two groups was evaluated according to the KPS score. The health status was improved by 46.51 % in research group and 25.71 % in control group, with a notable difference (p<0.05) (Table 4).
| Indicators | Research group (n=86) | Control group (n=70) | χ2/t | p |
|---|---|---|---|---|
| Improved | 40 (46.51) | 18 (25.71) | ||
| Stabilized | 37 (43.02) | 32 (45.71) | ||
| Deteriorated | 9 (10.47) | 20 (28.57) | ||
| Improvement rate | 40 (46.51) | 18 (25.71) | 7.146 | 0.008 |
Table 4: Improvement of Health Status in the Two Groups
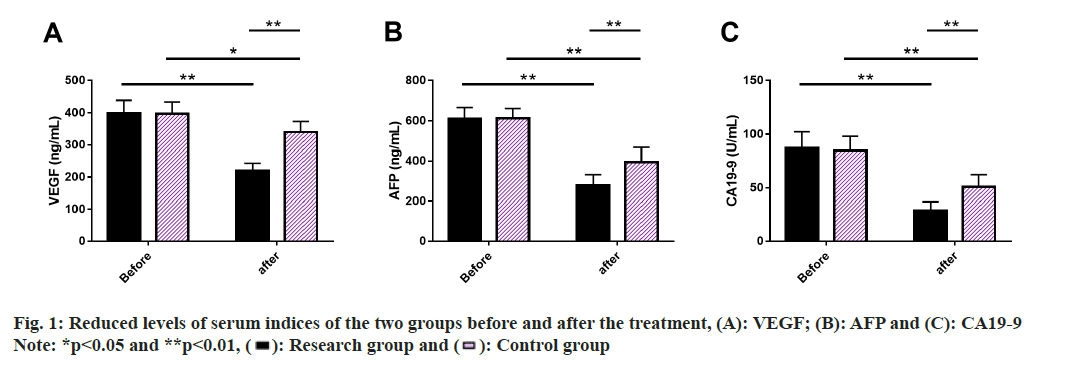
Serum indices of the two groups were evaluated, where we performed ELISA to measure serum VEGF, AFP and CA19-9 levels. These indices did not differ much between the two groups prior to treatment (p>0.05), but they all showed a significant decrease after treatment (p<0.05), with lower posttreatment levels in research group than in control group (p<0.05) (fig. 1).
Finally, 1 y OS of two groups was studied and compared by drawing survival curves. 1 y OS was found to be 79.07 % in research group and 50.00 % in control group, with a statistical significance (p<0.05) (fig. 2).
Most PLC patients are diagnosed with advanced HCC, with as many as 900 000 new cases and about 830 000 deaths worldwide annually, resulting in a great medical burden for patients and society[15,16]. In addition, patients with advanced HCC are often accompanied by typical clinical symptoms like liver pain, which has a negative impact on their daily life and work[17]. Therefore, focusing on the treatment of advanced HCC patients and providing better treatment options are of great clinical implications to improve treatment outcomes, alleviate clinical symptoms and improve quality of life.
Many researchers have provided optimized schemes for the FOLFOX regimen in the treatment of advanced PLC patients. For example, the application of FOLFOX in combination with Astragalus polysacharin injection which is effective in enhancing clinical efficacy, physical status and safety in patients with gastric cancer[18]. Wu et al.[19] reported that the FOLFOX regimen combined with recombinant human adenovirus type 5 for HCC patients was significantly beneficial in reducing the risk of metastasis and recurrence, and increasing the progression-free survival, with a favorable safety profile. Li et al.[20] also pointed out that HAIC with FOLFOX can significantly prolong the diseasefree survival of HCC patients with microvascular invasion, and the toxicity is tolerable. Currently, there are limited analytical studies on the efficacy of FOLFOX which includes OXA, FU and CF with HAIC regimen in advanced PLC patients, so this study made a relevant attempt. The efficacy evaluation revealed an evidently higher overall effective rate in research group and control group (70.93 % vs. 42.86 %), suggesting a certain effect of the FOLFOXHAIC regimen in enhancing the efficacy in advanced PLC patients and a better treatment response of this therapy compared with FOLFOX through the routine intravenous infusion, which is consistent with the research results of Si et al.[21]. This may be attributed to the fact that the FOLFOX-HAIC regimen may be more helpful in reducing tumor load, maintaining residual liver proliferation and preventing tumor progression in advanced PLC patients[22]. On the other hand, the therapeutic advantage of this regimen may also be related to its high anti-tumor effect under prolonged infusion during HAIC therapy[23]. As to complications, the statistics showed markedly lower incidences of nausea, vomiting, fatigue, fever, abnormal liver function and thrombocytopenia in research group compared with control group, which means that the FOLFOX-HAIC regimen has a certain preventive effect on the occurrence of complications in patients with advanced PLC. Although the extended duration of chemotherapy infusion under HAIC may elicit well-tolerated hepatotoxicity, patients receiving such treatments tend to have a lower risk of liver function decompensation and surgery-related death[24,25]. In the study of Lai et al.[25], the FOLFOXHAIC regimen not only a significant antitumor therapeutic effect in patients with advanced HCC with high-risk characteristics, but also has a certain safety profile, similar to our results. After the KPS score test, the health improvement rate of research group was also confirmed to be higher than that of control group, suggesting that the FOLFOX-HAIC regimen can effectively improve the health status of advanced PLC patients. Subsequently, ELISA quantization denoted that levels of serum indices such as VEGF, AFP and CA19-9 were significantly lower in research group than in control group, indicating that the FOLFOX-HAIC regimen can significantly inhibit serum VEGF, AFP and CA19-9 levels in advanced PLC patients, thus playing a synergistic role in anti-liver cancer to a certain extent. The survival curve analysis further determined an obviously higher 1 y OS rate in research group vs. control group (79.07 % vs. 50.00 %), which indicates that the FOLFOXHAIC regimen is helpful to improve prognosis and OS in advanced PLC patients. In a randomized phase III trial, FOLFOX-HAIC regimen was also shown to have a significant effect in prolonging OS, similar to our findings[26]. In another study, the FOLFOX-HAIC regimen was reported to be cost-effective in patients with large unresectable HCC[27].
Conclusively, this study confirms that FOLFOXHAIC (OXA+FU+CF) is better than TACE alone in advanced PLC patients in terms of efficacy, complications, improvement in health status, serum levels of tumor-related markers, and 1 y OS, with obvious clinical advantages in all the above dimensions, which is worthy of clinical promotion. Our findings also provide an optimized choice for the clinical management of patients with advanced PLC, which is conducive to improving the clinical outcomes of such patients.
Funding:
This research was funded by the Basic and Clinical Cooperation Research Promotion Program of the Third Affiliated Hospital of Anhui Medical University (Grant no: 2022sfy002).
Conflict of interests:
The authors declared no conflict of interests.
References
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology 2021;73:4-13.
[Crossref] [Google Scholar] [PubMed]
- Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 2015;24(1):1-17.
[Crossref] [Google Scholar] [PubMed]
- Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng J, et al. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer 2021;148(5):1051-65.
[Crossref] [Google Scholar] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391(10127):1301-14.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 2020;9(6):682-720.
[Crossref] [Google Scholar] [PubMed]
- Kloeckner R, Galle PR, Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology 2021;73:137-49.
[Crossref] [Google Scholar] [PubMed]
- Guo J, Yu Z, Sun D, Zou Y, Liu Y, Huang L. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol Cancer 2021;20(1):1-17.
[Crossref] [Google Scholar] [PubMed]
- On J, Park HA, Yoo S. Development of a prediction models for chemotherapy-induced adverse drug reactions: A retrospective observational study using electronic health records. Eur J Oncol Nurs 2022;56:102066.
[Crossref] [Google Scholar] [PubMed]
- Li JH, Xie XY, Zhang L, Le F, Ge NL, Li LX, et al. Oxaliplatin and 5-fluorouracil hepatic infusion with lipiodolized chemoembolization in large hepatocellular carcinoma. World J Gastroenterol 2015;21(13):3970-7.
[Crossref] [Google Scholar] [PubMed]
- Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin vs. doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31(28):3501-8.
[Crossref] [Google Scholar] [PubMed]
- He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, et al. Hepatic artery infusion chemotherapy using mFOLFOX vs. transarterial chemoembolization for massive unresectable hepatocellular carcinoma: A prospective non-randomized study. Chin J Cancer 2017;36(1):83.
[Crossref] [Google Scholar] [PubMed]
- Hsu SJ, Xu X, Chen MP, Zhao ZY, Wang Y, Yin X, et al. Hepatic arterial infusion chemotherapy with modified FOLFOX as an alternative treatment option in advanced hepatocellular carcinoma patients with failed or unsuitability for transarterial chemoembolization. Acad Radiol 2021;28:157-66.
[Crossref] [Google Scholar] [PubMed]
- Pinero F, Marciano S, Fernandez N, Silva J, Anders M, Zerega A, et al. Intermediate-advanced hepatocellular carcinoma in Argentina: Treatment and survival analysis. World J Gastroenterol 2019;25(27):3607-18.
[Crossref] [Google Scholar] [PubMed]
- Lian Q, Liu C, Chen F, Wang B, Wang M, Qiao S, et al. Orthopedic therapeutic surgery for bone metastasis of liver cancer: Clinical efficacy and prognostic factors. Front Surg 2022;9:1-10.
[Crossref] [Google Scholar] [PubMed]
- Peng D, Cai Y, Chen G, Hou M, Luo X, Dongzhi Z, et al. Efficacy and safety of apatinib vs. sorafenib/placebo in 1st-line treatment for intermediate and advanced primary liver cancer: A systematic review and meta-analysis. Front Pharmacol 2023;14:1-11.
- Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77(6):1598-606.
[Crossref] [Google Scholar] [PubMed]
- Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 2017;34(2):153-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang D, Zheng J, Ni M, Wu J, Wang K, Duan X, et al. Comparative efficacy and safety of Chinese herbal injections combined with the FOLFOX regimen for treating gastric cancer in China: A network meta-analysis. Oncotarget 2017;8(40):68873-89.
[Crossref] [Google Scholar] [PubMed]
- Wu K, You N, Zheng L. Effects of recombinant human adenovirus type 5 combined with transarterial chemoembolization on postoperative metastasis and recurrence of hepatocellular carcinoma patients. J Gastrointest Oncol 2021;12(6):2999-3007.
[Crossref] [Google Scholar] [PubMed]
- Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang CK, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: A multicenter, phase III, randomized study. J Clin Oncol 2023;41(10):1898-908.
[Crossref] [Google Scholar] [PubMed]
- Si T, Huang Z, Khorsandi SE, Ma Y, Heaton N. Hepatic arterial infusion chemotherapy vs. transarterial chemoembolization for unresectable hepatocellular carcinoma: A systematic review with meta-analysis. Front Bioeng Biotechnol 2022;10:1-13.
[Crossref] [Google Scholar] [PubMed]
- Zhuo W, Li A, Yang W, Duan J, Min J, Wei J. Case report: Hepatic artery infusion chemotherapy after stage I ALPPS in a patient with huge HCC. Front Surg 2021;8:1-7.
[Crossref] [Google Scholar] [PubMed]
- Gao J, Zhen R, Liao H, Zhuang W, Guo W. Pharmacokinetics of continuous transarterial infusion of 5-fluorouracil in patients with advanced hepatocellular carcinoma. Oncol Lett 2018;15(5):7175-81.
[Crossref] [Google Scholar] [PubMed]
- Li M, Zhang K, He J, Zhang W, Lv T, Wang L, et al. Hepatic arterial infusion chemotherapy in hepatocellular carcinoma: A bibliometric and knowledge-map analysis. Front Oncol 2023;12:1-11.
[Crossref] [Google Scholar] [PubMed]
- Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer 2022;174:68-77.
[Crossref] [Google Scholar] [PubMed]
- Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil and leucovorin vs. transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol 2022;40(2):150-60.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Zeng X, Peng Y, Tan C, Wan X. Cost-effectiveness analysis of hepatic arterial infusion chemotherapy of infusional fluorouracil, leucovorin and oxaliplatin vs. transarterial chemoembolization in patients with large unresectable hepatocellular carcinoma. Front Pharmacol 2022;13:1-7.
[Crossref] [Google Scholar] [PubMed]
 ): Research group and (
): Research group and ( ): Control group
): Control group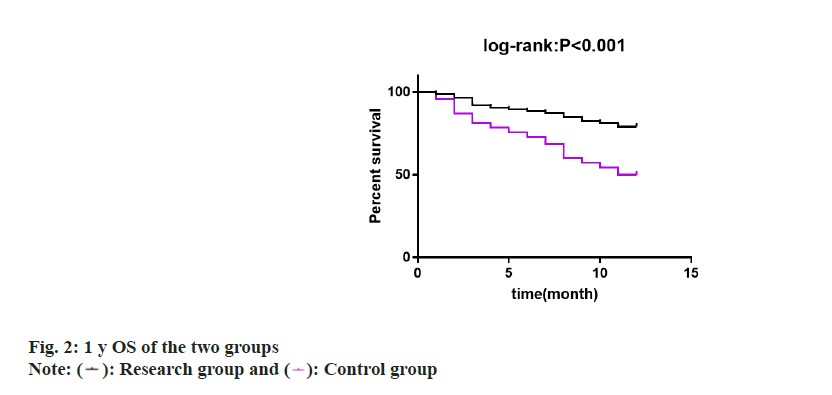
 ): Research group and (
): Research group and ( ): Control group
): Control group



