- *Corresponding Author:
- Lin Tan
Department of Gynecology, Shijiazhuang Great Wall Hospital of Integrated Traditional Chinese and Western Medicine, Shijiazhuang, Hebei Province 050000, China
E-mail: thl9077@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “91-97” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study mainly reports the efficacy of Nei Yi formula with diphereline in patients with ovarian endometriomas after surgery and its influence on ovarian functioning. 115 ovarian endometriomas individuals treated in our hospital were selected from December 2019 to March 2023 and were grouped into a control group (n=55) treated and research group (n=60). Control group was treated with diphereline while the research group was treated with Nei Yi formula on the basis of the control group. The treatment efficacy was evaluated comparing the symptom improvement of dysmenorrhea, breast tenderness, anal swelling and dyspareunia. Further, echocardiographic indices such as antral follicle count, resistance index of ovarian stromal flow, and ovarian cyst diameter were assessed. Additionally, ovarian functioning using serum estradiol, luteinizing hormone and follicle stimulating hormone was compared. Similarly, inflammatory indices such as tumor necrosis factor-alpha and interleukin-10 were also evaluated for comparative analysis. The data revealed a statistically higher overall response rate of treatment in the research group vs. the control group. Besides, the research group exhibited markedly reduced symptom improvement scores, resistance index, ovarian cyst diameter, ovarian function indices and tumor necrosis factor-alpha after treatment, lower than the baseline and the control group; while antral follicle count and interleukin-10 increased significantly after treatment, higher than the baseline and the control group. The above demonstrates the definite efficacy of Nei Yi formula with diphereline in the treatment of endometriomas patients after surgery, which can significantly ameliorate clinical symptoms and echocardiographic indices and facilitate the repair of ovarian function and the suppression of serum inflammation.
Keywords
Nei Yi formula, diphereline, ovarian endometriomas, postoperative effect, ovarian function
Endometriosis is a common gynecological condition which can affect 10 % of women at childbearing age. It is pathologically characterized by the appearance of endometrial glands and stroma at extrauterine (ectopic) sites[1,2]. It carries a high risk of recurrence, a long course of disease and can be clinically classified into peritoneal, ovarian and deep-infiltrating types[3,4]. Among them, Ovarian Endometriomas (EMA) are the most common clinical manifestation of endometriosis, accounting for approximately 17 %-44 % of total cases which is carrying 35 % risk of developing benign ovarian cysts[5,6]. EMA not only damages the adjacent ovarian cortical tissue, but it also affects the normal functioning of the ovaries, causing excessive secretion of estrogen, driving the occurrence of inflammatory reaction in the body and further aggravating the condition[7,8]. Until today, radical surgery has been the support of treatment for EMA, but it may have varying degrees of negative effects on ovarian function, which is difficult to cure and is associated with a high risk of postoperative recurrence[9,10]. Therefore, seeking an effective treatment strategy to improve the postoperative outcome of EMA is beneficial to control the patients' disease, the improvement of ovarian function and the alleviation of clinical symptoms.
Diphereline which is a Gonadotropin Releasing hormone-agonist (GnRh-a) and estrogen suppressor, can reduce ovarian hormone levels by inhibiting pituitary secretion of gonadotropin and strengthen the ectopic endometrial atrophy, thus playing a preventive role in postoperative recurrence[11,12]. In addition, GnRh-a can be used in the treatment of adenomyosis to assist high-intensity focused ultrasound, reduce the volume of uterine and adenomyotic lesions and promote symptomatic relief[13]. However, a rat model experiment of perimenopausal symptoms shows that GnRh-a intervention may cause perimenopausal symptoms, which may limit the use and efficacy of this therapy[14]. In the theory of Traditional Chinese Medicine (TCM), EMA is divided into “lump in the abdomen” and "abdominal pain during menstruation", with qi-stagnation and blood stasis as its common syndrome type. It is considered to be mainly related to liver-qi depression and emotional distress, so its treatment is primarily based on promoting qi and blood circulation, tonifying kidney and qi, clearing heat and promoting diuresis[15].
Nei Yi formula is mainly composed of fructus mume, Lindera aggregata, Salvia miltiorrhiza, common motherwort, peach kernel, oyster, Eupolyphaga sinensis (steleophaga), Rhizoma Corydalis, seaweed, Fritillary thunberg bulb, hawthorn, Cattail pollen, Trogopterus dung and Ligusticum wallichii. This composition can be used for the treatment of EMA and all the components play an important role in promoting qi and blood circulation[16].
There are currently limited clinical studies on the treatment of EMA with Nei Yi formula combined with diphereline. This study mainly verifies the clinical advantages of this combination therapy in EMA treatment, aiming to provide new insights into the clinical management of EMA.
Materials and Methods
General information:
115 EMA patients who received treatment at our hospital between December 2019 and March 2023 were selected and were divided into two groups namely, control group (n=55) and research group (n=60). The control group was treated with diphereline, while the research group was treated with diphereline along with Nei Yi formula. No notable inter-group difference was determined in general data (p>0.05). This study received approval from the ethics committee approval from our hospital and informed consent was obtained from each participant.
Inclusion criteria:
All the patients who met the EMA diagnostic criteria according to the revised-American Fertility Society (r-AFS) classification; patients with qi-stagnation and blood stasis syndrome; patients with varying degrees of dysmenorrhea, dyspareunia, pelvic pain and irregular menstruation; patients without echo mass in the uterine adnexa; patients with high echoic spots inside and patients having typical EMA signs diagnosed through Brightness (B) scan-ultrasonography were included in the study.
Exclusion criteria:
Patients having previous history of abdominal surgery and reproductive system surgery; patients with sexually transmitted diseases or genitourinary infections; patients with autoimmune disorders and deficiency or blood coagulation dysfunction; patients who were allergic to the drugs in this study and patients with mental illness and cognitive impairment were excluded from the study.
Treatment methods:
The control group was treated with diphereline. All the patients were given 3.75 mg intramuscular injection of diphereline on the 5th day of menstruation, once every 4 w. On this basis, the research group was additionally treated with Nei Yi formula which included 15 g of common motherwort, 20 g of oyster, 10 g of Eupolyphaga sinensis, 20 g of peach kernels, 15 g of Rhizoma Corydalis, 15 g of seaweed, 25 g fructus mume, 15 g of Lindera aggregata, 10 g of Fritillary thunberg, 15 g of hawthorn, 20 g of Salvia miltiorrhiza, 15 g of Cattail pollen, 15 g of Trogopterus dung and 15 g of Ligusticum wallichii. The formula was decocted in water to 200 ml for oral administration and single dose was given every. Patients were instructed to take it twice (morning and evening) warmly on an empty stomach since the 5th d of menstruation. This treatment was discontinued during menstruation and resumed after menstruation. Both the groups were treated continuously for 6 mo.
Detection indicators:
Clinical effectiveness: It is evaluated by grades like marked effectiveness, response and non-response. Reduction of resolved clinical symptoms and signs, disappearance of masses by ultrasonography and normal menstruation are considered as marked response. Response corresponds to the improvement of clinical symptoms and signs, disappearance of masses shown by ultrasonography and normal menstruation. Similarly, non-response refers to no improvement or aggravation of signs and symptoms, and appearance of new cysts indicated by ultrasonography.
Overall Response Rate (ORR)=sum of marked effectiveness+effective cases/total number of cases×100
Safety: The improvement of major symptoms such as dysmenorrhea, breast tenderness, anal swelling, dyspareunia before and after treatment were evaluated according to the diagnostic efficacy criteria of TCM diseases, with 1-4 points for each of the symptom. Higher scores suggest more serious symptoms.
Ultrasonographic indices: These indices include changes in Antral Follicle Count (AFC), Resistance Index (RI) of ovarian stromal flow and ovarian cyst diameter were determined by color Doppler ultrasonography.
Ovarian function: Fasting peripheral venous blood was collected before and after treatment for the quantification of serum Estradiol (E2), Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH). Quantification process was carried out using Enzyme-Linked Immunosorbent Assay (ELISA) technique.
Inflammatory indices: ELISA was performed to determine the levels of inflammatory indices such as Tumor Necrosis Factor-Alpha (TNF)-α and Interleukin (IL)-10.
Statistical analysis:
This study employed GraphPad Prism version 7.0 for statistical analysis and p<0.05 was considered as the statistically significant. The mean±standard deviation (x±s) was used to statistically describe measured data. Independent sample t-tests and paired t-tests were employed for inter- and intra-group comparisons, respectively. Count data, described as n (%), was compared between groups using Chi-square (χ²) test.
Results and Discussion
Clinical information of EMA patients in the two groups was compared. Baseline characters such as age, disease course, cyst diameter, gravidity, r-AFS staging, smoking history and alcohol abuse history were compared where control and research groups showed no evident differences (p>0.05) (Table 1).
| Factors | Control group (n=55) | Research group (n=60) | χ²/t | p |
|---|---|---|---|---|
| Age (y) | 28.96±5.37 | 29.52±5.14 | 0.571 | 0.569 |
| Disease course (mo) | 27.15±11.35 | 27.42±11.25 | 0.123 | 0.902 |
| Cyst diameter (cm) | 7.42±0.86 | 7.27±1.16 | 0.782 | 0.436 |
| Gravidity (times) | 1.44±0.50 | 1.57±0.59 | 1.269 | 0.207 |
| r-AFS staging (I-II/III-IV) | 27/28 | 25/35 | 0.639 | 0.424 |
| History of smoking (yes/no) | 15/40 | 22/38 | 1.160 | 0.281 |
| History of alcoholism (yes/no) | 18/37 | 18/42 | 0.099 | 0.753 |
Table 1: Clinical information of EMA patients in two groups.
Treatment response among the EMA patients of the two groups was compared. The ORRs of the control and research groups was found to be 72.73 % and 91.67 %, respectively, suggesting better therapeutic efficacy in the research group (p<0.05) (Table 2).
| Factors | Control group (n=55) | Research group (n=60) | χ² | p |
|---|---|---|---|---|
| Marked response | 25 (45.45) | 35 (58.33) | ||
| Response | 15 (27.27) | 20 (33.33) | ||
| Non-response | 15 (27.27) | 5 (8.33) | ||
| ORR | 40 (72.73) | 55 (91.67) | 7.165 | 0.007 |
Table 2: Clinical efficacy of EMA patients in two groups.
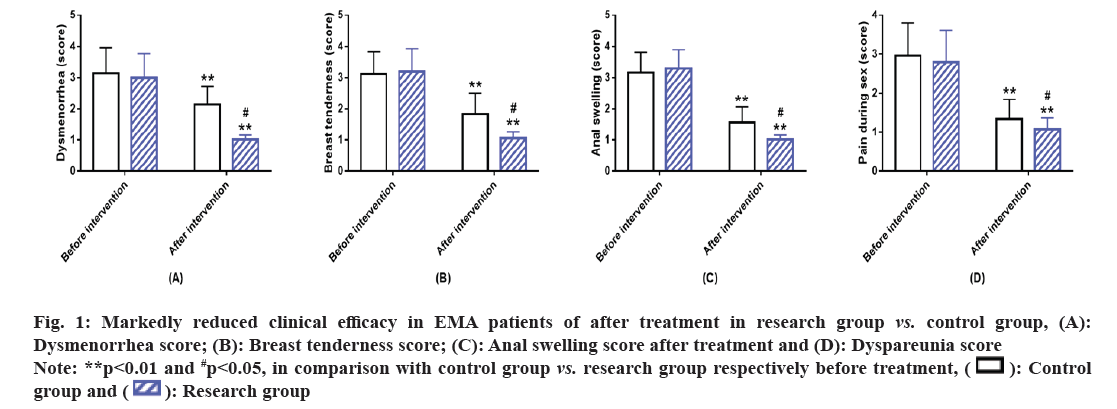
Symptom improvement in two groups of EMA patients was comparatively analyzed between the two groups. The improvement of clinical symptoms such as dysmenorrhea, breast tenderness, anal swelling and dyspareunia revealed no marked inter-group difference before treatment (p>0.05). After treatment, the clinical symptom scores reduced statistically in both the groups, with even lower scores in the research group (p<0.05) (fig. 1).
Fig. 1: Markedly reduced clinical efficacy in EMA patients of after treatment in research group vs. control group, (A):
Dysmenorrhea score; (B): Breast tenderness score; (C): Anal swelling score after treatment and (D): Dyspareunia score.
Note: **p<0.01 and #p<0.05, in comparison with control group vs. research group respectively before treatment,  .
.
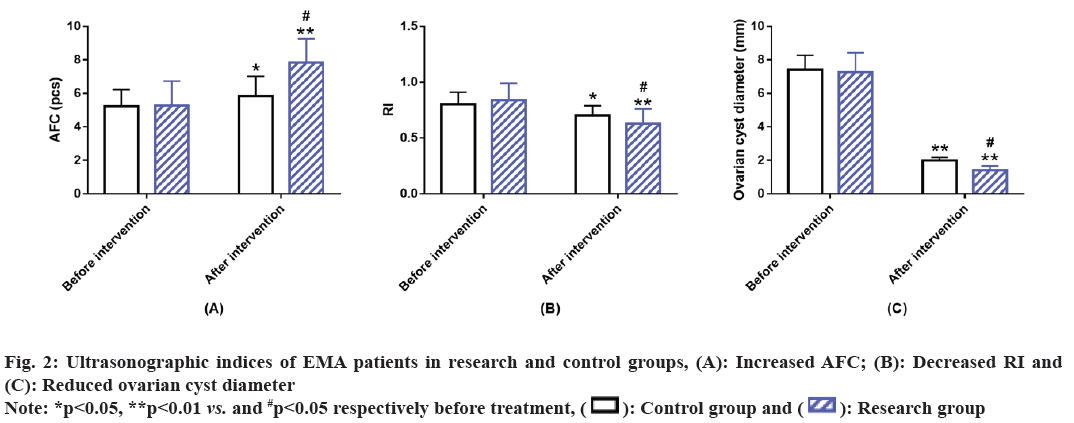
Ultrasonographic indices of EMA patients in two groups were compared. After analyzing the ultrasonographic indices such as AFC, RI and ovarian cyst diameter, it was found that there was no significant difference between the research and the control groups before intervention (p>0.05). However, after treatment AFC of both groups increased significantly, while RI and ovarian cyst diameter decreased significantly. In comparison with the control group, AFC was higher while RI and ovarian cyst diameter were lower in the research group (p<0.05) (fig. 2).
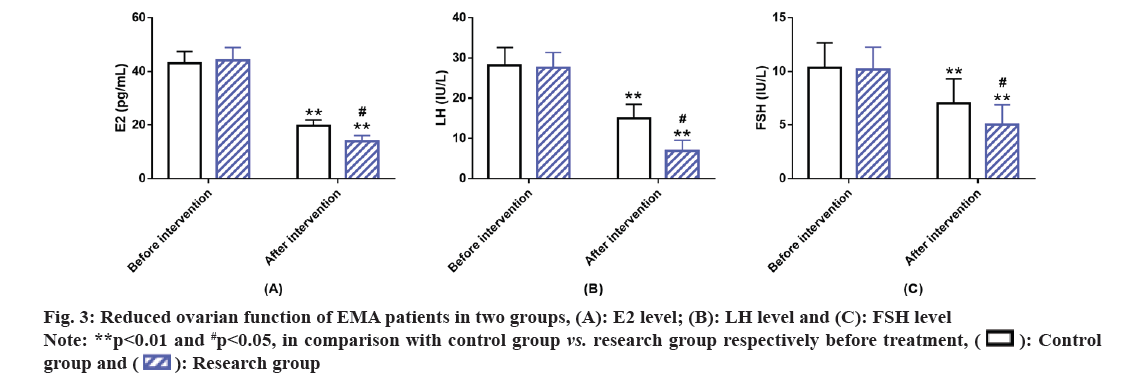
Ovarian functioning in the two groups of EMA patients was evaluated. The testing of ovarian function indices such as E2, LH and FSH revealed no marked inter-group differences before intervention (p>0.05). Marked reduction in these ovarian function indices was observed in both the groups after intervention, with lower levels in the research group than in the control group (p<0.05) (fig. 3).
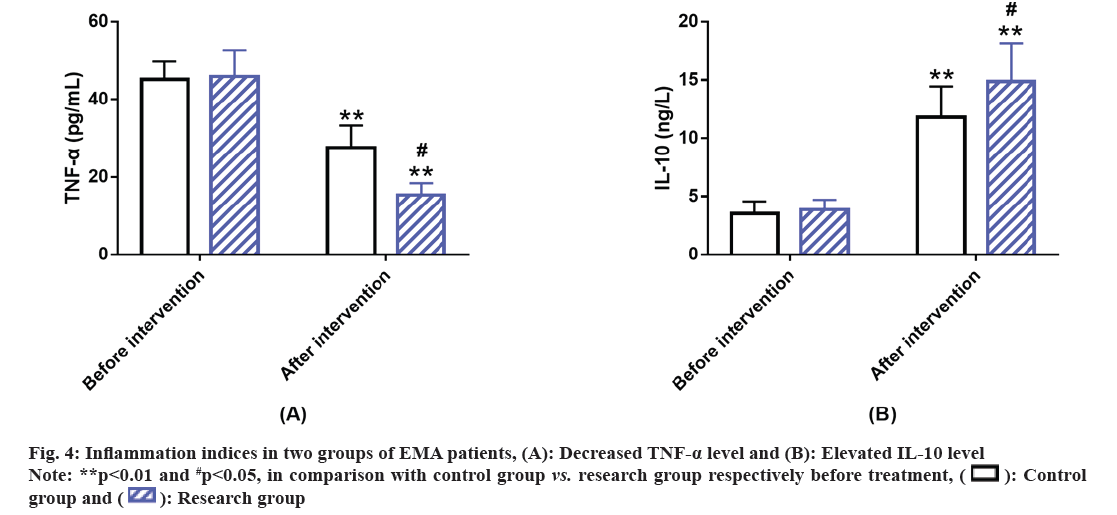
Further, inflammation indices in the EMA patients of the two groups were compared. We assessed the expression levels of inflammatory indices like TNF-α and IL-10 before and after the intervention; we found no evident inter-group difference before intervention (p>0.05). However, after intervention, the level of TNF-α in both the groups decreased with a lower level in the research group vs. control group; while the post-interventional IL-10 level of both groups increased, with an even higher level in the research group (p<0.05) (fig. 4).
Although EMA is a benign inflammatory reproductive disease, it can cause dysmenorrhea, chronic fatigue, low fertility, chronic pelvic pain and bladder symptoms such as ozostomia and abdominal distension, etc., in female patients, which negatively affects their normal life and even fertility[17,18]. Therefore, it is of great value to explore new effective treatment strategies for the clinical treatment of EMA to relieve patients' symptoms and facilitate their restoration of normal life and fertility.
In this study, the ORR of the research group was found to be markedly superior to that of the control group (91.67 % vs. 72.73 %), suggesting that Nei Yi formula+diphereline has a better clinical effect on EMA. This may be attributed to the effective ingredients present in the Nei Yi formula. fructus mume can strengthen vital meridian and relieve pain, Lindera aggregata can warm the middle warmer to dispel cold and promote qi circulation helping to relieve pain. Salvia miltiorrhiza can help to reinforce the liver and kidney and promote blood circulation to restore menstrual flow while common motherwort can activate blood to dissolve stasis, clear away heat and toxic materials. Similarly, peach kernel plays the role of promoting blood circulation and removing blood stasis and, Eupolyphaga sinensis is capable of dispelling stasis and eliminating addiction[19-22].
The combination of the above mentioned Chinese herbal medicines and diphereline can play a synergistic therapeutic effect on EMA patients to a certain extent. In the research of Gao et al.[23], the intervention of Salvia miltiorrhiza-containing Chinese herbs combined with GnRh-a for postoperative treatment of EMA helps to improve the postoperative effect and reduce the risk of postoperative recurrence, with significant safety, similar to this study. Another study shows that Chinese herbal compound prescription has a significant effect on EMA-related infertility, which is conducive to improving the pregnancy rate while ensuring certain safety[24]. Subsequently, the evaluation of clinical symptom relief revealed that the scores of clinical symptoms such as dysmenorrhea, breast tenderness, anal swelling and dyspareunia reduced statistically in the research group and were lower when compared with the control group, suggesting that the combination of Nei Yi formula and diphereline can significantly alleviate the clinical symptoms of patients after EMA treatment with a relief effect superior to diphereline intervention alone.
Furthermore, the ultrasonographic indices such as AFC, RI and ovarian cyst diameter improved significantly in the research group after intervention, with higher AFC and lower RI and ovarian cyst diameter compared with the control group, indicating that the treatment of EMA with Nei Yi formula combined with diphereline can significantly increase the number of sinus follicles, improve ovarian follicular atresia and microcirculation, which is of great help to the restoration of ovarian function. The testing results of E2, LH and FSH showed obviously reduced indices after treatment in the research group were lower vs. the control group, suggesting that EMA patients can achieve significant inhibition of estrogen secretion and repair of ovarian reserve function after receiving the intervention of Nei Yi formula with diphereline. After the detection of inflammatory indices such as TNF-α and IL-10, the research group was found to present markedly inhibited TNF-α and elevated IL-10 than the control group, indicating the ability of Nei Yi formula with diphereline to validly alleviate the inflammatory microenvironment of EMA patients. IL-10 may mediate the pathogenesis of EMA and inhibit TNF-α by regulating the Nuclear Factor Kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) pathway in endometrial cells, thus weakening the induction of IL-6 synthesis by TNF-α[25]. Yu et al.[26] pointed out in their study that the effect of Nei Yi formula on EMA can significantly reduce the weight of endometriosis tissue and the number of peritoneal macrophages, which may be related to its up-regulation of IL-10 level to inhibit inflammatory reaction and the growth of endometriosis tissue, similar to our research results.
In conclusion, the combination of Nei Yi formula and diphereline can significantly improve efficacy in post-surgical EMA patients, which can improve the curative effect, relieve clinical symptoms, repair ovarian function and inhibit serum inflammatory reaction, providing new path and reference for efficacy optimization in EMA patients.
Author's contributions:
Huijing Dong and Xiaochao Sun contributed equally to this work and are the co-first authors. Huijing Dong and Xiaochao Sun designed the research and wrote the first version of the manuscript. Huijing Dong, Xiaochao Sun, Qian Liu and Haolin Tan contributed to conceiving the research and analyzing the data. Huijing Dong and Xiaochao Sun conducted the analysis and provided guidance for the research. All the authors reviewed and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer 2009;100(8):1315-9.
[Crossref] [Google Scholar] [PubMed]
- Koninckx PR, Ussia A, Adamyan L, Tahlak M, Keckstein J, Wattiez A, et al. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract Res Clin Obstet Gynaecol 2021;71:14-26.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Qu P. Factors associated with ovarian endometriosis malignancy and its recurrence in Chinese women. J Obstet Gynaecol 2019;39(8):1148-53.
[Crossref] [Google Scholar] [PubMed]
- Guidozzi F. Endometriosis-associated cancer. Climacteric 2021;24(6):587-92.
[Crossref] [Google Scholar] [PubMed]
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet 2016;134(1):3-7.
[Crossref] [Google Scholar] [PubMed]
- Gałczynski K, Jozwik M, Lewkowicz D, Semczuk-Sikora A, Semczuk A. Ovarian endometrioma-A possible finding in adolescent girls and young women: A mini-review. J Ovarian Res 2019;12(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update 2014;20(2):217-30.
[Crossref] [Google Scholar] [PubMed]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometriosis. Endocr Rev 2019;40(4):1048-79.
- Nowak-Psiorz I, Ciećwież SM, Brodowska A, Starczewski A. Treatment of ovarian endometrial cysts in the context of recurrence and fertility. Adv Clin Exp Med 2019;28(3):407-13.
[Crossref] [Google Scholar] [PubMed]
- Sweed MS, Makled AK, El-Sayed MA, Shawky ME, Abd-Elhady HA, Mansour AM, et al. Ovarian reserve following laparoscopic ovarian cystectomy vs. cyst deroofing for endometriomas. J Minim Invasive Gynecol 2019;26(5):877-82.
[Crossref] [Google Scholar] [PubMed]
- Stamatiades GA, Carroll RS, Kaiser UB. GnRH-A key regulator of FSH. Endocrinology 2019;160(1):57-67.
[Crossref] [Google Scholar] [PubMed]
- Zheng Q, Mao H, Xu Y, Zhao J, Wei X, Liu P. Can postoperative GnRH agonist treatment prevent endometriosis recurrence? A meta-analysis. Arch Gynecol Obstet 2016;294(1):201-7.
[Crossref] [Google Scholar] [PubMed]
- Pang LL, Mei J, Fan LX, Zhao TT, Li RN, Wen Y. Efficacy of high-intensity focused ultrasound combined with GnRH-A for adenomyosis: A systematic review and meta-analysis. Front Public Health 2021;9:1-12.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Wang H, Dong Z, Liu J, Qin Z, Bao M, et al. GnRH-a-induced perimenopausal rat modeling and black cohosh preparations' effect on rat's reproductive endocrine. Front Endocrinol 2021;12:1-16.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Shi Y, Xu L, Wang Z, Wang Y, Shi W, et al. Traditional Chinese medicine prescription Guizhi Fuling pills in the treatment of endometriosis. Int J Med Sci 2021;18(11):2401-8.
[Crossref] [Google Scholar] [PubMed]
- Zheng W, Wu J, Gu J, Weng H, Wang J, Wang T, et al. Modular characteristics and mechanism of action of herbs for endometriosis treatment in Chinese medicine: A data mining and network pharmacology-based identification. Front Pharmacol 2020;11:1-43.
[Crossref] [Google Scholar] [PubMed]
- Alkatout I, Wedel T, Maass N. Kombinierte Therapie der Endometriose: Radikal und schonend zugleich [Combined treatment of endometriosis: Radical yet gentle]. Aktuelle Urol 2018;49(1):60-72.
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers 2018;4(1):1-9.
[Crossref]
- Chen Z, He L, Tang W, Gu Q, Wang Y, Wang K, et al. Clinical effect of wumei bolus on ulcerative colitis: A meta-analysis. Heliyon. 2023;9(5):1-14.
[Crossref] [Google Scholar] [PubMed]
- Miao LL, Zhou QM, Peng C, Liu ZH, Xiong L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: A comprehensive overview. Biomed Pharmacother 2019;117:1-12.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Liu Z, Wu Y. Acupuncture combined with traditional Chinese medicine and drug therapy for the treatment of cerebral infarction (phlegm-blood stasis syndrome) and carotid atherosclerotic plaque: A preliminary randomized controlled study. Appl Bionics Biomech 2022:1-7.
[Crossref] [Google Scholar] [PubMed]
- Liang X, Wang Q, Jiang Z, Li Z, Zhang M, Yang P, et al. Clinical research linking traditional Chinese medicine constitution types with diseases: A literature review of 1639 observational studies. J Tradit Chin Med 2020;40(4):690-702.
[Crossref] [Google Scholar] [PubMed]
- Gao Q, Shen L, Jiang B, Luan YF, Lin LN, Meng FC, et al. Salvia miltiorrhiza-containing Chinese herbal medicine combined with GnRH agonist for postoperative treatment of endometriosis: A systematic review and meta-analysis. Front Pharmacol 2022;13:1-11.
[Crossref] [Google Scholar] [PubMed]
- Dong P, Ling L, Hu L. Systematic review and meta-analysis of traditional Chinese medicine compound in treating infertility caused by endometriosis. Ann Palliat Med 2021;10(12):12631-42.
[Crossref] [Google Scholar] [PubMed]
- Tagashira Y, Taniguchi F, Harada T, Ikeda A, Watanabe A, Terakawa N. Interleukin-10 attenuates TNF-alpha-induced interleukin-6 production in endometriotic stromal cells. Fertil Steril 2009;91:2185-92.
[Crossref] [Google Scholar] [PubMed]
- Yu C, Liu Y, Peng X. IL-8 and IL-10 levels in endometriosis and regulated role of neiyifang on them. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000;20(8):603-5.
[Google Scholar] [PubMed]