- *Corresponding Author:
- Li Wang
Department of Joint Surgery, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi 830001, China
E-mail: 1712023006@stu.sqxy.edu.cn
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “25-32” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This research compared the efficacy of apixaban and low molecular weight heparin in patients undergoing hip arthroplasty and their influence on coagulation function and postoperative deep vein thrombosis. We enrolled 114 patients undergoing hip arthroplasty and assigned them into control group (n=50) and research group (n=64) treated with low molecular weight heparin and apixaban, respectively. The coagulation function which included platelets, prothrombin time, activated partial thromboplastin time, incidence of postoperative deep vein thrombosis, levels of deep vein thrombosis and their associated serum markers like c-reactive protein, d-dimer, homocysteine, and their safety were evaluated and compared between the two groups. The results showed no significant differences in pre- and post-treatment platelets, prothrombin time, activated partial thromboplastin time between research group and control group. The research group was observed with statistically lower postoperative deep vein thrombosis and overall complication rates, as well as lower c-reactive protein, d-dimer and homocysteine levels compared before and after treatment. The results show that although apixaban has no significant effect on blood coagulation in patients undergoing hip arthroplasty. It can effectively prevent postoperative deep vein thrombosis while significantly inhibiting c-reactive protein, d-dimer and homocysteine with fewer adverse reactions.
Keywords
Apixaban, low molecular weight heparin, hip arthroplasty, coagulation function, deep venous thrombosis, therapeutic effect
Hip Arthroplasty (HA) is a surgical operation for hip joint injuries, which mainly uses prostheses to replace the damaged hip joint completely or partially in order to achieve the therapeutic purpose[1]. This surgical procedure has been applied to multiple medical settings such as hip pain, joint stiffness, hip fracture, and hip osteoarthritis, exerting a significant improvement effect on patients' hip function, quality of life, low back pain and hip pain[2-4]. According to epidemiological statistics, the number of patients undergoing HA is increasing by about 20 % with at least 1 million HA surgeries performed worldwide each year[5,6]. Although HA plays a certain role in pain relief and joint function recovery, there are inevitable postoperative complications and persistent injuries, which hinder the recovery efficacy of patients with this surgical method[7]. Therefore, it is necessary to find effective counter measures for the above problems to ensure the restoration effect of this surgical approach, which is of great clinical significance for improving the efficacy of patients. Venous Thromboembolism (VTE), an inducement that leads to increased risk of death and treatment cost, is a common complication after HA[8]. This event generally includes postoperative Deep Vein Thrombosis (DVT) and pulmonary embolism, with the risk varying widely from 1 %-2 % to 60 % due to population heterogeneity and differences in treatment and diagnosis[9]. VTE refers to abnormal blood flow that may be related to factors such as blood hypercoagulability[10]. Therefore, it is crucial to give anticoagulants to patients undergoing HA. Apixaban (API) is an orally activated coagulation Factor X (FXa) inhibitor that can be used in a variety of thromboembolic diseases and is also currently used in HA to deal with DVT and other adverse events[11]. As an anticoagulant for HA patients, it has a series of advantages, such as predictable pharmacokinetics and pharmacodynamics, few drug-food interactions, and relatively wide treatment window[12]. Russell et al.[13] reported that API can significantly reduce the incidence of DVT after total hip/knee arthroplasty, with a better preventive effect than enoxaparin. Besides API, Low Molecular Weight Heparin (LMWH) is also the standard drug which is used to prevent DVT[14]. Previous studies have shown that LMWH has more advantages in terms of safety and effectiveness than unfractionated heparin, with strong anticoagulant and anti-inflammatory effects, few adverse reactions and high bioavailability[15]. It can play an antithrombotic role by improving coagulation function and reducing the injury of vascular endothelial cells related to thrombosis[16]. This paper primarily compares the curative effect of API vs. LMWH in the treatment of patients undergoing HA and their influence on coagulation function and postoperative DVT, aiming to provide new insights for DVT prevention after HA.
Materials and Methods
General information:
This research enrolled 114 patients undergoing HA between December 2018 and December 2021, and assigned them into 2 groups, control group (n=50) and research group (n=64) treated with LMWH and API, respectively, based on the drug therapy they received.
On an average, patients in the control group were aged (56.94±7.66) y, with a ratio of male to female of 27:23 while the data in the research group was 32:32 and (57.81±8.94) y, respectively. The two groups showed no significant difference in general data (p>0.05), with clinical comparability.
This research was conducted strictly adhering to the declaration of Helsinki, has obtained approval from the Ethics Committee of Qingpu Branch of Zhongshan Hospital affiliated to Fudan University Shanghai, as well as informed consent from all the patients.
Eligibility criteria:
The patients who met the study indications, patients who underwent HA surgery for the 1st time with smooth operation, and those whose communication and cognitive ability were normal, have been included in this study.
Similarly, those patients with coagulation dysfunction, malignant tumors, vital organ dysfunction, allergy to API or LMWH and patients who had mental illness or communication disorders were excluded.
Treatment:
Both the groups were given routine symptomatic treatment and supportive treatment according to their conditions.
Patients in the control group were given the 1st dose of enoxaparin (Yubo Biological Co., Ltd., E0180000) at 12 h after surgery with a subcutaneous injection of 5000 IU, followed by a daily subcutaneous injection of 5000 IU for 5 w. While, patients in the research group were treated with API tablets (Hubei Youngxin Pharmaceutical Technology Co., Ltd., JX20140275) 12 h after surgery, 2.5 mg/time, twice a day, for 5 consecutive.
Measurement indicators:
Coagulation function: Blood samples were collected before and 3 d after surgery to measure Platelets (PLT), Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) with a CA1500 coagulometer produced by Sysmex Shanghai Ltd.
Postoperative DVT incidence rate: The incidence of postoperative DVT in both groups was recorded and calculated. The diagnostic criteria of DVT were observed. It was observed that the lumen cannot be completely closed after venous compression; no echo or low echo in the lumen and no or little blood flow signal in local veins was seen. Doppler showed no blood flow or no change in blood flow spectrum with respiration.
DVT-associated serum parameters: Before and after 3 d of surgery, C-reactive Protein (CRP) was measured using Enzyme-Linked Immunosorbent Assay (ELISA), while D-Dimer (D-D) and Homocysteine (Hcy) were detected by an automatic coagulation analyzer.
Safety: The incidence of adverse events like gastrointestinal reactions, increased incision bleeding, abnormal liver function, eosinophilia, and thrombocytopenia were recorded in both the groups.
Statistical analysis:
In this study, statistical software Statistical Package for Social Sciences (SPSS) 19.0 version was used for data analysis. The number of patients/ percentage (n/%) and mean±Standard Error of the Mean (SEM) were used to represent the counting data and quantitative data, respectively. Chi-square (χ2) test was used for inter-group comparisons of counting data. The measurement data between 2 groups were compared by t-test, and by paired t-test before and after treatment where p<0.05 was the significant.
Results and Discussion
Baseline data of patients undergoing HA was studied, for which characteristics like gender, mean age, Body Mass Index (BMI), disease type, HA type, course of disease, span of hospital stay, etc. were compared between groups and found no statistical differences (p>0.05) (Table 1).
| Factors | Control group (n=50) | Research group (n=64) | χ2/t | p |
|---|---|---|---|---|
| Gender (male/female) | 27/23 | 32/32 | 0.18 | 0.672 |
| Average age (years) | 56.94±7.66 | 57.81±8.94 | 0.548 | 0.585 |
| BMI (kg/m2) | 25.93±3.44 | 26.11±3.68 | 0.267 | 0.79 |
| Disease type (femoral neck fracture/femoral head necrosis/osteoarthritis) | 40/7/3 | 47/12/5 | 0.67 | 0.715 |
| Type of HA (total HA/hemiarthroplasty) | 39/11 | 49/15 | 0.033 | 0.856 |
| Course of disease (d) | 40.92±9.60 | 40.13±12.68 | 0.366 | 0.715 |
| Span of hospital stay (d) | 12.12±2.25 | 12.47±2.48 | 0.778 | 0.438 |
Table 1: Baseline Data of Patients Undergoing HA (n, mean±SEM)
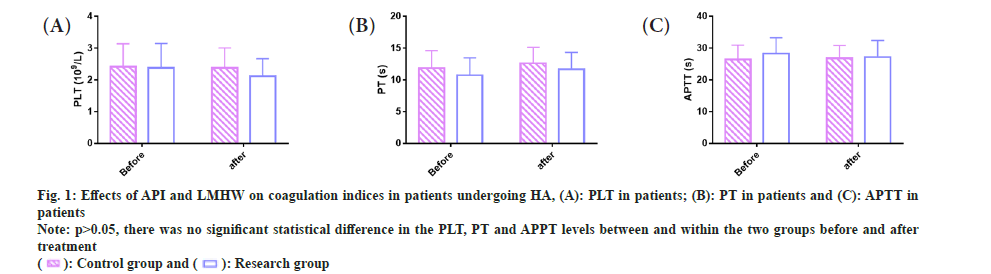
Coagulation function in patients undergoing HA was compared. We examined coagulation indicators in both groups to study the impact of API and LMWH on coagulation function of patients undergoing HA. The pre- and post-treatment PLT of the control group were (2.42±0.72) 109/l and (2.38±0.63) 109/l, vs. (2.38±0.77) 109/l and (2.12±0.55) 109/l in the research group, respectively. The pre- and post-treatment PT were (11.87±2.73) s and (12.62±2.53) s in the control group while (10.74±2.74) s and (11.69±2.65) s in the research group, respectively. The pretreatment APTT levels of the control and research groups were (26.40±4.55) s and (26.86±4.00) s, respectively, while the post-treatment levels were (28.28±5.01) s and (27.18±5.24) s, respectively.
The above data revealed no significant differences in PLT, PT, and APTT between and within the groups before and after treatment (p>0.05) (fig. 1).
Fig 1: Effects of API and LMHW on coagulation indices in patients undergoing HA, (A): PLT in patients; (B): PT in patients and (C): APTT in patients
Note: p>0.05, there was no significant statistical difference in the PLT, PT and APPT levels between and within the two groups before and after treatment 
Incidence of postoperative DVT in HA patients was observed. In the control group, the patients of femoral, popliteal and gastrocnemius vein thrombosis and embolism were 1, 2, 5, and 0, respectively; it can be seen that the cases of gastrocnemius vein thrombosis were the most frequent, followed by popliteal vein thrombosis and femoral vein thrombosis. In the research group, femoral, popliteal, and gastrocnemius vein thrombosis and embolism were found in 0, 2, 0, and 0 patients, respectively, with popliteal vein thrombosis being the most commonly found adverse event. The research group presented statistically lower incidence rates of femoral, popliteal and gastrocnemius vein thrombosis, embolism, and obviously lower total DVT incidence than the control group (3.13 % vs. 16.00 %) (p<0.05) (Table 2).
| Categories | Control group (n=50) | Research group (n=64) | χ2 | p |
|---|---|---|---|---|
| Femoral vein thrombosis | 1 (2.00) | 0 (0.00) | - | - |
| Popliteal vein thrombus | 2 (4.00) | 2 (3.13) | - | - |
| Gastrocnemius vein thrombosis | 5 (10.00) | 0 (0.00) | - | - |
| Embolism | 0 (0.00) | 0 (0.00) | - | - |
| Total | 8 (16.00) | 2 (3.13) | 5.815 | 0.016 |
Table 2: Effects of API and LMWH on the Incidence of Postoperative DVT in Patients Undergoing Ha, N (%)
Univariate analysis of the occurrence of postoperative DVT in patients undergoing HA was observed. In order to understand the underlying factors influencing the development of postoperative DVT in patients undergoing HA, we performed a univariate analysis. All the 114 patients undergoing HA were regrouped to a DVT group (n=10) and a non-DVT group (n=104) according to the occurrence of DVT after surgery. Univariate analysis showed that gender, average age, disease type, type of HA, course of disease, and span of hospital stay did not have any significant effect on the development of DVT after HA (p>0.05), while BMI had a significant impact on postoperative DVT in patients undergoing HA (p=0.009) (Table 3).
| Factors | DVT group (n=10) | Non-DVT group (n=104) | χ2/t | p |
|---|---|---|---|---|
| Gender (male/female) | 6/4 | 53/51 | 0.298 | 0.585 |
| Average age (<60/≥60) years | 3/7 | 56/48 | 3.221 | 0.073 |
| BMI (<28/≥kg/m2) | 4/6 | 81/23 | 6.903 | 0.009 |
| Disease type (femoral neck fracture/femoral head necrosis/osteoarthritis) | 5/3/2 | 82/16/6 | 4.797 | 0.091 |
| Type of HA | 7/3 | 81/23 | 0.322 | 0.57 |
| Course of disease (<45/≥45) d | 5/5 | 70/34 | 1.214 | 0.271 |
| Span of hospital stay (<12/≥15) d | 5/5 | 76/28 | 2.362 | 0.124 |
Table 3: Univariate Analysis of the Occurrence of Postoperative DVT in Patients Undergoing Ha
| Categories | Control group (n=50) | Research group (n=64) | χ2 | p |
|---|---|---|---|---|
| Gastrointestinal reactions | 2 (4.00) | 1 (1.56) | - | - |
| Increased incision bleeding | 2 (4.00) | 1 (1.56) | - | - |
| Abnormal liver function | 2 (4.00) | 1 (1.56) | - | - |
| Eosinophilia | 1 (2.00) | 0 (0.00) | - | - |
| Thrombocytopenia | 1 (2.00) | 0 (0.00) | - | - |
| Total | 8 (16.00) | 3 (4.68) | 4.120 | 0.042 |
Table 4: Effects of API and LMWH on the Safety of Patients Undergoing Ha, n (%)
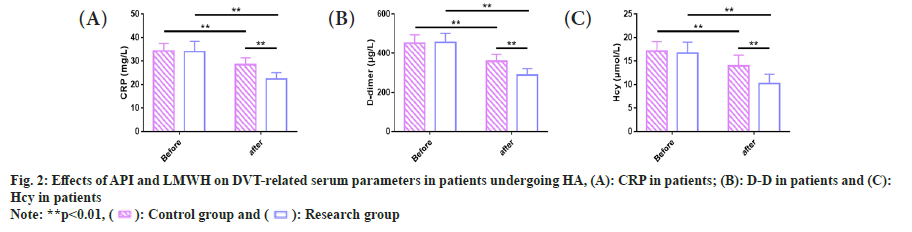
Similarly, serum markers associated with DVT in patients undergoing HA was observed. We examined DVT-related serum parameters in the two groups. The pre- and post-treatment CRP levels were (34.34±3.23) mg/l and (28.50±2.84) mg/l in the control group while (33.96±4.46) mg/l and (22.33±2.84) mg/l in the research group, respectively. The pre-treatment D-D levels of the control and research groups were (451.60±42.23) μg/l and (455.18±46.24) μg/l, respectively, while the post-treatment levels were (358.97±35.02) μg/l and (290.30±31.92) μg/l, respectively. The pre- and post-treatment Hcy levels in the control group were (17.12±2.03) μmol/l and (13.98±2.25) μmol/l, respectively, vs. (16.66±2.40) μmol/l and (10.20±2.09) μmol/l in the research group. Through comparison, we found similar CRP, D-D and Hcy levels in the two groups before treatment (p<0.01). After treatment, the three serum markers decreased significantly in both groups (p<0.01), and were even lower in the research group (p<0.01) (fig. 2).
There was no significant difference in CRP levels between the two groups of patients undergoing HA before treatment (p>0.05), but the CRP level in the research group significantly reduced compared with the control group after treatment (p<0.05).
The level of D-D was not statistically significant between the two groups of patients undergoing HA before treatment (p>0.05). After treatment, the D-D level in the research group significantly reduced, compared with the control group (p<0.05).
The Hcy levels of two groups of patients undergoing HA, showed no statistical significance before treatment (p>0.05), but the Hcy level in the research group significantly reduced compared with the control group after treatment (p<0.05).
Furthermore, safety of the patients undergoing HA was studied. We evaluated the safety of both groups of patients to analyze the impact of two interventions on patient safety. The results showed that the number of cases of gastrointestinal reactions, increased incision bleeding, abnormal liver function, eosinophilia, and thrombocytopenia in the research group was 1, 1, 1, 0, and 0, respectively, with a total incidence of 4.68 %, while that in the control group was 2, 2, 2, 1, and 1, respectively, with a total incidence of 16.00 %. By comparing the incidence rates of all these adverse reactions and the overall incidence were determined to be markedly lower in the research group than in the control group (p<0.05) (Table 4).
HA, which is a surgical treatment commonly used in orthopedics to treat hip diseases such as femoral neck fracture, femoral head necrosis, and osteoarthritis, can relieve joint pain, dysfunction and improve patients’ quality of life[17]. The risk of post-HA DVT is 40 %-60 %, which not only induces unbearable pain in patients, but also increases the hospitalization time and brings a heavy medical burden to them[18]. Hence, giving anticoagulants to patients to prevent DVT after HA is very important.
API, an oral antithrombotic preparation, comparable to enoxaparin is suitable for both knee replacement and HA patients, effectively reducing the incidence of DVT and the risk of postoperative bleeding[19,20]. Enoxaparin, is a kind of LMWH that can only be administered subcutaneously, can inhibit FXa by binding to antithrombin, thus playing an antithrombotic role[21]. Enoxaparin has been shown to reduce the incidence of postoperative symptomatic DVT in patients without increasing major bleeding events[22]. Our research results showed non-significant differences in PLT, PT and APTT between groups before and after treatment, similar to the results of Dong et al.[11], suggesting that API and LMWH have similar anticoagulant effects on patients. Besides, the research group (3.13 %) had a markedly lower incidence rate of femoral, popliteal and gastrocnemius vein thrombosis as well as embolism than the control group (16.00 %), indicating a better preventive action of API against DVT than LMWH, which is consistent with the findings of Jiang et al.[23]. According to Aryal et al.[24], API is advantageous over LMWH in the prevention of thrombosis in patients undergoing knee replacement or HA, mainly manifested in a lower incidence of DVT, which may be associated with the lower risk of bleeding of the former.
In order to further explore the potential risk factors affecting the development of DVT in patients undergoing HA, we conducted a relevant univariate analysis, which found that BMI had a significant impact on the development of DVT in patients undergoing HA, ≥28 kg/m2 which significantly increased the risk of DVT in such patients. Obesity has also been shown to be a risk factor for DVT after HA in several studies[25,26], that seems similar to our findings. This is closely related to factors such as increased operation time, more limited postoperative mobility, ineffective mechanical prophylaxis, and excessive secretion of procoagulant inflammatory markers in obese patients[27]. Shaka et al.[28] further reported that although the mortality rate of obese patients undergoing HA surgery was comparable to that of non-obese patients, the morbidity rate and the risk of perioperative complications were higher.
On the other hand, we evaluated the effects of both the treatments on DVT-related serum parameters to analyze the potential molecular mechanism of the two treatment modalities in the prevention of DVT. CRP, D-D and Hcy levels in the research group decreased after treatment and were significantly lower compared with the control group, indicating that API may play an anti-DVT role by inhibiting the secretion of CRP, D-D and Hcy, with a more potent inhibitory effect on these DVT-related indicators than LMWH. Finally, we assessed the safety of both the groups in terms of adverse events such as gastrointestinal reactions, increased incision bleeding, abnormal liver function, eosinophilia and thrombocytopenia. The results showed that the overall incidence of adverse reactions in patients undergoing HA with API intervention were significantly lower (4.68 % vs. 16.00 %), indicating that API was well tolerated.
The uniqueness of this study is to comprehensively analyze the clinical efficacy and safety of API and LMWH in the treatment of patients with HA from the perspectives of blood coagulation, incidence of postoperative DVT, DVT related serum indices and incidence of adverse reactions. It is confirmed that API has a more prominent positive effect on the prevention of DVT and other adverse reactions after HA, which may be related to the strong inhibition of this therapy on DVT-related serum indicators such as CRP, D-D and Hcy, providing a reliable basis and new insights for the postoperative management of patients undergoing HA. But still, there are some limitations. First of all, as it is a small single-center research, there may be information bias in the samples collected that may have some influence on the universality of the research results. Further, we have not analyzed the risk factors of DVT in patients undergoing HA, which can be supplemented to provide certain guidance for the prevention of postoperative DVT. The future studies will focus on the above mentioned limitations for supplementary analysis.
To sum up, although API has no significant effect on blood coagulation in patients undergoing HA, compared with LMWH it can significantly reduce the incidence of postoperative DVT, and decrease levels of DVT associated serum parameters such as CRP, D-dimer, Hcy, with a significantly lower incidence of adverse reactions, which deserves clinical popularization.
Conflict of interests:
The authors declared no conflict of interest.
References
- Meng Y, Deng B, Liang X, Li J, Li L, Ou J, et al. Effectiveness of self-efficacy-enhancing interventions on rehabilitation following total hip replacement: A randomized controlled trial with six-month follow-up. J Orthop Surg Res 2022;17(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet 2007;370(9597):1508-19.
[Crossref] [Google Scholar] [PubMed]
- Choi EJ, Choi YJ, Lee SW, Choi YM, Ri HS, Park JY, et al. Effect of anesthetic method on incidence of delirium after total hip replacement arthroplasty in South Korea: A population-based study using National Health Insurance claims data. Korean J Anesthesiol 2020;73(1):36-43.
[Crossref] [Google Scholar] [PubMed]
- Eguchi Y, Iida S, Suzuki C, Shinada Y, Shoji T, Takahashi K, et al. Spinopelvic alignment and low back pain after total hip replacement arthroplasty in patients with severe hip osteoarthritis. Asian Spine J 2018;12(2):325-34.
[Crossref] [Google Scholar] [PubMed]
- Katano H, Ozeki N, Kohno Y, Nakagawa Y, Koga H, Watanabe T, et al. Trends in arthroplasty in Japan by a complete survey, 2014-2017. J Orthop Sci 2021;26(5):812-22.
[Crossref] [Google Scholar] [PubMed]
- Ferguson RJ, Palmer AJ, Taylor A, Porter ML, Malchau H, Glyn-Jones S. Hip replacement. Lancet 2018;392(10158):1662-71.
[Crossref] [Google Scholar] [PubMed]
- Sang W, Xue S, Xu Y, Liu Y, Zhu L, Ma J. Bikini incision increases the incidence of lateral femoral cutaneous nerve injury in direct anterior approach hip arthroplasty: A prospective ultrasonic, electrophysiological, and clinical study. J Arthroplasty 2021;36(10):3463-70.
[Crossref] [Google Scholar] [PubMed]
- Song W, Ma T, Cheng Q, Wen P, Wu J, Hao L, et al. Global research status and trends in venous thromboembolism after hip or knee arthroplasty from 1990 to 2021: A bibliometric analysis. Front Med 2022;9:1-12.
[Crossref] [Google Scholar] [PubMed]
- Deng W, Huo L, Yuan Q, Huang D, Li Q, Tian W. Risk factors for venous thromboembolism in patients with diabetes undergoing joint arthroplasty. BMC Musculoskelet Disord 2021;22(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Kim YH, Park JW, Kim JS. Chemical thromboprophylaxis is not necessary to reduce risk of thromboembolism with tranexamic acid after total hip arthroplasty. J Arthroplasty 2017;32(2):641-4.
[Crossref] [Google Scholar] [PubMed]
- Dong X, Liu X, Liu Y, Jiang L, Zhang H, Liu B. Clinical efficacy of conventional heparin anticoagulation combined with apixaban in the treatment of patients with cerebral venous thrombosis and its effect on serum D-dimer and FIB expression. Comput Math Methods Med 2021;2021:4979210.
[Crossref] [Google Scholar] [PubMed]
- Frost C, Garonzik S, Shenker A, Barrett YC, LaCreta F. Apixaban single-dose pharmacokinetics, bioavailability, renal clearance, and pharmacodynamics following intravenous and oral administration. Clin Pharmacol Drug Dev 2021;10(9):974-84.
[Crossref] [Google Scholar] [PubMed]
- Russell RD, Huo MH. Apixaban and rivaroxaban decrease deep venous thrombosis but not other complications after total hip and total knee arthroplasty. J Arthroplasty 2013;28(9):1477-81.
[Crossref] [Google Scholar] [PubMed]
- Lee JK, Lee KB, Kim JI, Park GT, Cho YC. Risk factors for deep vein thrombosis even using low-molecular-weight heparin after total knee arthroplasty. Knee Surg Relat Res 2021;33(1):29.
[Crossref] [Google Scholar] [PubMed]
- Zhang P, Qu Y, Tu J, Cao W, Hai N, Li S, et al. Applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis in patients with COVID-19 and treatment with low molecular weight heparin. J Clin Ultrasound 2020;48(9):522-6.
[Crossref] [Google Scholar] [PubMed]
- Sadowski R, Gadzala-Kopciuch R, Buszewski B. Recent developments in the separation of low molecular weight heparin anticoagulants. Curr Med Chem 2019;26(1):166-76.
[Crossref] [Google Scholar] [PubMed]
- Yu X, Wu Y, Ning R. The deep vein thrombosis of lower limb after total hip arthroplasty: What should we care. BMC Musculoskelet Disord 2021;22(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Wainwright TW, Gill M, McDonald DA, Middleton RG, Reed M, Sahota O, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations. Acta Orthop 2020;91(1):3-19.
[Crossref] [Google Scholar] [PubMed]
- Rooney T, Barrack RL, Clohisy JC, Nunley RM, Lawrie CM. Is apixaban safe and effective for venous thromboembolism prophylaxis after primary total hip and total knee arthroplasties? J Arthroplasty 2021;36(7S):S328-31.
[Crossref] [Google Scholar] [PubMed]
- Ali Hasan M, Azeez Alsaadi M, Tahseen Mehsen J. Effectiveness of apixaban vs. enoxaparin in preventing wound complications and deep venous thrombosis following total knee replacement surgery: A retrospective study. Int J Clin Pract 2021;75(10):1-5.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Chang D, Chui J, Cao J, Negus J. The efficacy and cost-effectiveness of enoxaparin vs. rivaroxaban in the prevention of venous thromboembolism following total hip or knee arthroplasty: A meta-analysis. J Orthop 2022;30:1-6.
[Crossref] [Google Scholar] [PubMed]
- Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e278S-325.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Meng J, Guo T, Zhao JN, Wang YC, Wang J, et al. Comparison of apixaban and low molecular weight heparin in preventing deep venous thrombosis after total knee arthroplasty in older adults. Yonsei Med J 2019;60(7):626-32.
[Crossref] [Google Scholar] [PubMed]
- Aryal MR, Pandit A, Ghimire S, Pathak R, Karmacharya P, Poudel DR, et al. Thromboprophylaxis with apixaban and the risk of pulmonary embolism in patients undergoing knee replacement surgery. J Community Hosp Intern Med Perspect 2015;5(4):27889.
[Crossref] [Google Scholar] [PubMed]
- Kim JS. Deep vein thrombosis prophylaxis after total hip arthroplasty in Asian patients. Hip Pelvis 2018;30(4):197-201.
[Crossref] [Google Scholar] [PubMed]
- Barg A, Henninger HB, Hintermann B. Risk factors for symptomatic deep-vein thrombosis in patients after total ankle replacement who received routine chemical thromboprophylaxis. J Bone Joint Surg Br 2011;93(7):921-7.
[Crossref] [Google Scholar] [PubMed]
- Sloan M, Sheth N, Lee GC. Is obesity associated with increased risk of deep vein thrombosis or pulmonary embolism after hip and knee arthroplasty? A large database study. Clin Orthop Relat Res 2019;477(3):523-32.
[Crossref] [Google Scholar] [PubMed]
- Shaka H, Ojemolon PE. Impact of obesity on outcomes of patients with hip osteoarthritis who underwent hip arthroplasty. Cureus 2020;12(10):1-7.
[Crossref] [Google Scholar] [PubMed]