- *Corresponding Author:
- Suzhen Wang
Department of Neurosurgery,Shandong Jiyang Public Hospital, Jinan 251400, China
E-mail: 3439055891@qq.com
| This article was originally published in a special issue: Special issue on “Drug Development and Human Health in China” | |
| Indian J Pharm Sci 2020:82(1)spl issue2;63-66 | |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study was aimed at evaluating the clinical efficacy of a combination of nimodipine and edaravone in the treatment of acute cerebral hemorrhage. A total of 180 patients diagnosed with acute cerebral hemorrhage and treated at the Shandong Jiyang Public Hospital were enrolled. The patients were divided into the control group and the research group with 90 patients in each group. Patients in the control group were treated with edaravone, while a combination of nimodipine and edaravone was administered to patients in the research group. Total therapeutic efficacy, neurologic impairment score, quality of life score, hematoma and edema volume were compared between both groups. Results obtained demonstrated that the total therapeutic efficacy of the research group was better than that of the control group (p<0.05). Patients’ hematoma and edema volume before and after treatment on comparison indicated that improvement in the research group after drug administration was significantly better than that of the control group (p<0.05). Moreover, there was a greater decrease in the neurologic impairment score of the research group than that of the control group (p<0.05). Application of a combination of nimodipine and edaravone in the treatment of acute cerebral hemorrhage could achieve greater clinical efficacy as well as significantly improve the therapeutic efficiency.
Keywords
Nimodipine, edaravone, drug combination, acute cerebral hemorrhage, clinical efficacy
Cerebral hemorrhage is a common but severe condition encountered in the neurology department with a relatively high incidence. Patient’s neurological function would be damaged to a varying degree when acute cerebral hemorrhage occurred, causing severe impacts on patients’ life quality or even threatening their lives[1,2]. Drugs such as edaravone and nimodipine have been a ray of light for those suffering from cerebral hemorrhage[3]. The key for improving the therapeutic efficacy lies in active and effective treatment regimens.
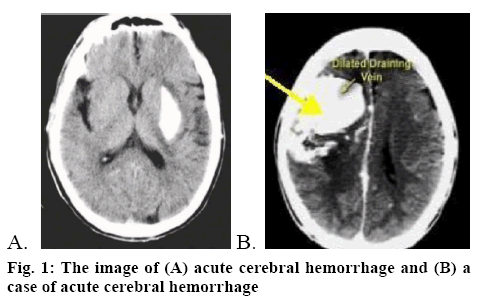
Acute cerebral hemorrhage (figure 1A) refers to bleeding caused by nontraumatic vessel rupture in the brain parenchyma. As a powerful hydroxyl radical scavenger, edaravone can penetrate the brain-blood barrier with high efficiency as well as protect neurons and promote their recovery. While, nimodipine is a calcium channel antagonist, which can effectively dilate the brain blood vessels[4] as well as reduce vasoconstriction, thus relieving vasospasm and improving the hemoperfusion of the brain. Present study involved evaluation of the clinical efficacy of a combination of nimodipine and edaravone in the treatment of acute cerebral hemorrhage. A total of 180 acute cerebral hemorrhage patients treated at the Shandong Jiyang Public Hospital from January 2016 to August 2018 were enrolled. All cases were in line with the diagnostic criteria of acute cerebral hemorrhage advanced by The Fourth Cerebrovascular Diseases Conference. Meanwhile, head CT or MRI test were adopted to confirm diagnosis (figure 1B). The inclusion criteria were as follows[5], the onset of cerebral hemorrhage was within 24 h, complicated with limb paralysis and the muscle strength was less than level IV; the patients and their family enjoyed the right of information and formal consent forms were signed; there was no cerebral hemorrhage caused by tumor, brain trauma, coagulation disorders and the like; there were no complications like infectious diseases and organ failure; no patient had malignant tumor or allergic to the medication used in this study. This research was approved by the ethics committee of the Shandong Jiyang Public Hospital. Patients were randomized into research group and control group, with 90 patients in each group. There were 50 male and 40 female patients in the research group, with an average age of 70.68±3.25 y. There were 46 male and 44 female patients in the control group, with an average age of 71.29±4.02 y. Data obtained from both groups such as sex, age, degree of education, family situation all were comparable.
Routine therapeutic approaches including in-time dehydration to lower the intracranial pressure, oxygen uptake, lipid metabolism mediation, maintenance treatment of water electrolyte balance and acidbase balance, as well as prevention and treatment of complications were adopted. Edaravone was given through intravenous drip, 30 mg of edaravone injection (Simcere Pharmaceutical Group, H20050280) was added into 100 ml of sodium chloride injection (0.9 %) for intravenous drip. Edaravone was administered twice a day, for a month.
Drug combination of nimodipine and edaravone was administered to the research group patients The therapeutic regimen of edaravone was the same as the control group. Moreover, 10 mg of nimodipine (Bayer Company, J20100002) was added into 100 ml of glucose injection (5 %) for intravenous drip, once a day. After 10 consecutive days of treatment, intravenous drip of nimodipine was changed into nimodipine tablet (Bayer Company, H20003010), 60 mg each time, three times a day. Similarly, the treatment was for a month.
The total clinical efficacy of both groups were observed and compared, which included 4 standards, namely basic recovery, excellent, valid and invalid. Of those, the total clinical efficacy was evaluated according to the National Institute of Health stroke scale (NIHSS) [6]. Basic recovery refers to a decline of 91-100 % in NIHSS after 2 w of treatment, while excellent refers to a decline of 50-91 % in NIHSS. If the NIHSS decreases by 21-50 %, then it is considered valid. If the NIHSS decreases less than 20 % or the condition is even worse, then it is considered invalid. Moreover, the NIHSS, activity of daily living (ADL) score, hematoma volume and edema volume were recorded.
Statistical analysis was performed using SPSS21.0. All quantitative data were expressed in the form of mean±standard deviation and comparisons were made using the t-test. Enumeration data were expressed in the form of natural number (n) +percent (%) and comparisons were made with chi-square test. P<0.05 represents the intergroup difference was of statistically significance.
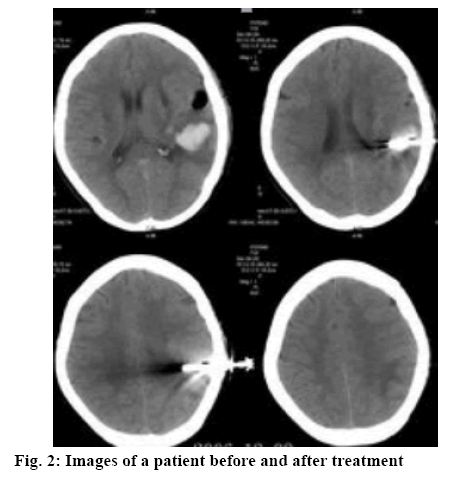
As shown in Table 1, the total clinical efficacy of the research group was significantly higher than that of the control group (p<0.05). The images of a patient before and after treatment were shown in figure 2. As shown in Table 2, no significant difference was observed regarding the hematoma volume and edema volume before treatment between both groups. After adopting different therapeutic regimens, the improvement in the research group was significantly better than that of the control group (p<0.05).
Table 1: Comparison of the Total Clinical Efficacy between Both Groups [N (%)]
| Groups | Basic recovery | Excellent | Valid | Invalid | Total clinical efficacy |
|---|---|---|---|---|---|
| Research group (n = 90) | 30 | 46 | 10 | 4 | 86 (95.56) |
| Control group (n = 90) | 14 | 32 | 28 | 16 | 74 (82.22) |
| X2 | 12.59 | ||||
| P | <0.05 |
Table 2: Comparisons of the Hematoma Volume and Edema Volume between Both Groups before and After Treatment
| Groups | Hematoma volume (ml) | Edema volume (ml) | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| Research group (n = 90) Control group (n = 90) |
20.50±3.32 | 9.70±2.29 | 6.56±2.53 | 2.53±4.19 |
| 21.66±3.20 | 16.55±1.98 | 6.19±2.78 | 4.60±3.92 | |
| t | 0.34 | 8.49 | 1.02 | 13.59 |
| P | >0.05 | <0.05 | >0.05 | <0.05 |
As shown in Table 3, no significant difference was observed regarding the NIHSS score and ADL score before treatment between both groups. After treatment, the scores of these two indicators of the research group was significantly better than that of the control group (p<0.05).
Table 3: Comparisons of the NIHSS Score and ADL Score between Both Groups before and After Treatment
| Groups | NIHSS score | ADL score | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| Research group (n = 90) Control group (n = 90) |
23.50±3.32 | 11.70±2.29 | 32.56±2.53 | 55.83±4.19 |
| 23.66±3.20 | 16.55±1.98 | 32.19±2.78 | 45.60±3.92 | |
| t | 0.34 | 8.49 | 1.02 | 13.59 |
| P | >0.05 | <0.05 | >0.05 | <0.05 |
Literature revealed that cerebral hemorrhage accounts for 20-30 % of all stroke cases, and the mortality rate in the acute phase is 30-40 %[7]. The causes of cerebral hemorrhage are mainly related to the pathological changes of the cerebrovascular circulation closely related to hyperlipidemia, diabetes, hypertension, aging of blood vessels and smoking. Patients suffered from cerebral hemorrhage often have sudden onset due to emotional agitation or strenuous exertion. The mortality is rather high in the early stage. Most survivors experience different degrees of dyskinesia, cognitive impairment, speech dysphagia and other sequelae. In the case of acute cerebral hemorrhage, most of the channel proteins are deactivated, which would alter the permeability of the cell membrane. Moreover, cellular apoptosis occurs and causes damage in brain tissue. During the treatment of acute cerebral hemorrhage, on one hand, it is necessary to effectively stabilize the hemorrhagic foci. On the other hand, it is necessary to actively control the secondary pathologic changes such as cerebral edema, limiting its further development.
As a typical neuroprotective agent, edaravone has low molecular weight as well as high lipophilicity. If the oxygen free radicals were neutralized at the same time, significant relief of inflammatory response in the brain tissue as well as of neuronal apoptosis, actively improving ischemia and hypoxia as well as promoting functional recovery of the neurons. The calcium antagonist, nimodipine could block the calcium channel on the cell membrane and inhibit the transfer of calcium into cell under hypoxia condition, thus lowering the cellular calcium concentration. Meanwhile, nimodipine could penetrate through the brain blood barrier and act in the brain tissue, exerting favorable neuron-protective effect. Furthermore, nimodipine could actively alleviate the vasospasm of brain vessels under hypoxia condition, thus promoting the micro-circulation in the position of bleeding and improving both oxygen and blood supply.
Results from this study indicated that the total therapeutic efficacy of the research group were obviously better than those of the control group (p<0.05). Results of Patients’ hematoma and edema volume before and after treatment indicated that the improvement in the research group after drug administration was significantly better than that of the control group (p<0.05). Moreover, there was a greater decrease in the neurologic impairment score of the research group than that of the control group (p<0.05). The above findings are in line with relevant reports[8-15].
To sum up, combination of nimodipine and edaravone in the treatment of acute cerebral hemorrhage could achieve good clinical efficacy. Edaravone could improve the permeability of the brain blood barrier, actively promote the removal of free radicals and alleviate the damage to cells and neurons. Nimodipie has high lipid solubility and could actively alleviate the vasospasm of brain vessels. Moreover, nimodipine could effectively improve the brain circulation, decrease the intracranial hematoma and promote the hematoma absorption. With the combination of these two drugs, the volume of hematoma and edema as well as the NIHSS were significantly decreased, thus the total therapeutic efficacy was obviously improved. This drug combination therapy is an important guarantee of higher life quality, with improved safety and acceptability. Therefore, drug combination of nimodipine and edaravone in the treatment of acute cerebral hemorrhage could bring more ideal results, which is worth clinical promotion.
References
- Yu JH. Clinical efficacy of Nimodipine combined with Edaravone in the treatment of 56 patients with acute cerebral hemorrhage. Chin J Modern Drug Appl 2016;10(22):143-4.
- Li HR. Protective effect of nimodipine on PC12 cell apoptosis induced by H2O2. Chin J Comparative Med 2017;27(04):14-19.
- Richard AB, Christopher JM, Philip DB, Alexander DS, James MM, Christopher PG. Risk stratification for ST segment elevation myocardial infarction in the era of primary percutaneous coronary intervention. World J Cardiol 2014;6(08):865-872.
- Hua Z, He XY, Zhuang SW, Wang J, Lai Y, Qi WG, Yao YA, et al. Clinical and procedural predictors of no-ref low in patients with acute myocardial infarction after primary percutaneous coronary intervention. World J Emerg Med, 2016;5(02):96-102.
- Wang YQ, Xu J, Guo R, Xu CX, Hao YM, Chen CF, et al. Therapeutic effect in patients with coronary heart disease based on information analysis from Traditional Chinese Medicine four diagnostic methods. J Tradit Chin Med 2017;34(01):34-41.
- Si KY, Li ZP. Effect of edaravone combined with nimodipine on the biochemical indexes of acute large area cerebral infarction. Chin J Pract Nerve Dis 2017;20(19):71-73.
- Lv JL, Arsen A. Clinical efficacy of edaravone combined with nimodipine in the treatment of hypertensive intracerebral hemorrhage. J Clin Med Literature 2018;5(02):143-144.
- Zhang M, He HW, Wang ZM, Xu ZH, Zhou NT, Tao ZX, et al. Diagnostic and prognostic value of minor elevated cardiac troponin levels for percutaneous coronary intervention-related myocardial injury: a prospective, single-center and double-blind study. J Biomed Res 2014;28(02):98-107.
- Li RJ, Hao PP, Chen YG, Zhang Y. Association of cystatin C level and cardiovascular prognosis for patients with preexisting coronary heart disease: A meta-analysis. Chin Sci Bull 2014;59(Z1):539-45.
- Demirel B, Bicakci U, Rizalar R, Alpaslan PF, Aydin O. Histopathological effects of mesenchymal stem cells in rats with bladder and posterior urethral injuries. Turk J Med Sci 2017;47(6):1912-9.
- Gao W, Wang Y, Basavanagoud B, Jamil MK. Characteristics studies of molecular structures in drugs. Saudi Pharm J 2017;25(4):580-6.
- Parisi L, Salerno M, Maltese A, Tripi G, Romano P, Di Folco A, et al. Paternal shift-working and sleep disorders in children affected by primary nocturnal enuresis. Acta Medica Mediterr 2017;33(3):481-4.
- Sadi B, Bayat M, Tajik P, Hashemi S. Citrinin detection by intensified fluorescence signal of a fret-based immunosensor using magnetic/silica core-shell. Saudi J Biol Sci 2018;25(1):171-7.
- Saheed S, Taofik S, Oladipo A, Tom A. Spondias mombin l. (Anacardiaceae) enhances detoxification of hepatic and macromolecular oxidants in acetaminophen-intoxicated rats. Pak J Pharm Sci 2017;30(6):2109-17.
- Yadav KD, Chaudhary AK, Verma AK. Bioavailability enhancement of partially water soluble solid medicament in traditional system of medicine. Indian J Pharm Sci 2017;79(5):667-73.