- *Corresponding Author:
- Zili Zhang
Department of Respiratory Medicine, Beijing Geriatric Hospital, Haidian, Beijing 100095, China
E-mail: zzl1990426@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “128-133” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study analyzes to elucidate the efficacy and safety of salmeterol/fluticasone propionate plus tiotropium bromide in the treatment of chronic obstructive pulmonary disease. 120 chronic obstructive pulmonary disease patients admitted between April 2019 to April 2022 were selected and were divided into research group (n=62) and control group (n=58). The research group received salmeterol/fluticasone propionate+tiotropium bromide therapy while the control group received tiotropium bromide. The efficacy, safety (headache, constipation, palpitation, and upper respiratory tract infection), pulmonary function, forced expiratory volume in 1 s/forced vital capacity ratio, serum inflammatory markers like c-reactive protein, interleukin-8, tumor necrosis factor-alpha and quality of life according to Saint George’s respiratory questionnaire were comparatively evaluated. The overall response rate was found to be markedly higher in the research group than the control group. A similar incidence of adverse reactions was determined in both the groups. In addition, the research groups showed higher post-interventional forced expiratory volume in 1 s/forced vital capacity, forced expiratory volume in 1 s/forced vital capacity ratio and Saint George’s respiratory questionnaire than the control group, as well as statistically lower c-reactive protein, interleukin-8 and tumor necrosis factor-alpha levels. The above results demonstrate excellent efficacy of salmeterol/fluticasone propionate plus tiotropium bromide for the treatment of chronic obstructive pulmonary disease, with a safety profile comparable to that of tiotropium bromide monotherapy with better effects improving pulmonary function, inhibiting serum inflammatory markers, and enhancing patients’ quality of life.
Keywords
Salmeterol, fluticasone propionate, tiotropium bromide, chronic obstructive pulmonary disease, forced expiratory volume, forced vital capacity
Chronic Obstructive Pulmonary Disease (COPD), which is a chronic and inflammatory respiratory condition, pathologically characterized by Chronic Airflow Limitation (CAL) and hyperinflammatory airway[1]. COPD patients usually present with symptoms such as nasal congestion, sore throat, purulent sputum, cough, fever, and chest tightness, with comorbidities such as diabetes, hypertension, coronary artery disease, interstitial lung disease and congestive heart failure[2,3]. Viruses, environmental pollution, airway bacteria, etc., may worsen COPD symptoms, resulting in impaired lung function, reduced Quality of Life (QOL), and increased risk of death[4-6]. According to statistics, respiratory failure is a major cause of death in patients with severe COPD. At present, the disease has become the 3rd leading cause of death in the world and kills 6 million people every year[7]. Currently COPD treatment is mainly based on medication and nondrug interventions (pulmonary rehabilitation therapy and self-management education), which has certain advantages in respiratory mechanics optimization and symptom relief, while the research on the latter is still in its infancy with some controversies[8,9]. Therefore from the perspective of medication, this study conducts relevant analysis hoping to contribute to the reduction of COPD mortality by exploring a relatively more ideal drug combination program.
Salmeterol/Fluticasone Propionate (SFP) is a compound preparation of long-acting Beta (β) 2 receptor agonist and inhaled corticosteroid, which is beneficial to improve Pulmonary Function (PF), health status and symptom relief, with a therapeutic effect better than long-acting β2 receptor agonists alone[10,11]. In the study of Steiropoulos et al.[12], the PF of COPD patients was significantly improved and dyspnea was obviously relieved after 6 mo and 12 mo of SFP treatment, without being influenced by comorbidities, suggesting certain clinical effectiveness of SFP in treating COPD. Moreover, SFP is superior to montelukast and fluticasone in the treatment of adolescent asthma, with the advantage of improving PF and reducing the risk of asthma attacks in the early morning[13]. Tiotropium Bromide (TB) is essentially a long-acting muscarine antagonist, which cannot only bind to Muscarinic acetylcholine (M3) receptors on airway smooth muscle cells, but also promote smooth muscle relaxation by inhibiting the effect of acetylcholine on muscarinic receptors, thus playing a pharmacological role in COPD[14,15]. As reported by Blair et al.[16], TB is not only effective and well tolerated in patients with moderate-tosevere COPD, but also cost-effective when combined with olodaterol
At present, there is limited research investigating the efficacy and safety of SFP plus TB in COPD. This study is hereby conducted and reported.
Materials and Methods
General information:
This research has been ratified by the Hospital's Ethics Committee. In this study, COPD patients admitted to our hospital from April 2019 to April 2022 were strictly screened out and enrolled as research participants according to the inclusion and exclusion criteria. Among them, 62 patients in the research group were treated with SFP+TB, while 58 patients in the control group were treated with TB. The two groups of patients were clinically compared and they showed no significant difference in baseline data (p>0.05).
Inclusion criteria:
Patients who were clinically diagnosed with COPD; with normal communication and cognitive ability; patients with no serious mental disorders, and no contraindications for the medication used in the study were included.
Exclusion criteria:
Those complicated with asthma or other lung diseases, pulmonary lesions caused by other diseases, immune system diseases, or other chronic and inflammatory diseases were excluded, as well as those with glucocorticoid therapy and other treatments in the 3 mo prior to treatment or inability to complete drug inhalation due to low inspiratory flow.
Treatment method:
All the patients received treatments such as routine oxygen inhalation, anti-infection, and bronchodilation, while ensuring airway patency. The control group was given TB monotherapy; patients were given TB powder for inhalation every morning, with an inhalation dose of 1 capsule/d. The research group was treated with SFP based on the above treatment, with an inhalation dose of 2 blisters/d, 2 times every day (once a blister). Both groups received a 3 mo treatment.
Observation endpoints:
Efficacy: The clinical symptoms and recovery of the two groups before and after treatment were compared and analyzed as the evaluation standard of treatment effectiveness. Cure means that the symptoms such as cough, expectoration, asthma, and lung wheezing sounds disappear completely, and the patient would be able to take care of themselves in daily life; a marked response is that the symptoms such as cough, expectoration, asthma, and lung wheezing sounds are obviously alleviated vs. before treatment, and the patient can basically take care of themselves in daily life; a response refers to improved symptoms such as cough, expectoration and asthma, reduced wheezing sound in the lungs, and the ability of the patient to partially take care of themselves in daily life; if the patient's symptoms such as cough, expectoration and asthma do not change significantly, the wheezing sound in the lungs is not reduced vs. before treatment, and the patient cannot take care of themselves in daily life, which is considered non-response. The Overall Response Rate (ORR) is the percentage of the sum of cure, marked response and response cases as a percentage of the total number of cases.
Safety: The number of adverse reactions such as headache, constipation, palpitation and Upper Respiratory Tract Infection (URTI) in both groups were observed and recorded, and the incidence was calculated.
PF indices: The Forced Expiratory Volume in 1 s (FEV1), Forced Vital Capacity (FVC) and the FEV1/FVC ratio were detected using a lung function detector.
Serum inflammatory markers: Fasting venous blood was collected from all patients, and serum was obtained after centrifugation for Enzyme-Linked Immunosorbent Assay (ELISA) quantification of the levels of inflammatory markers such as C-Reactive Protein (CRP), Interleukin-8 (IL-8) and Tumor Necrosis Factor-Alpha (TNF-α).
QOL: We employed the Saint George’s Respiratory Questionnaire (SGRQ) for the assessment of patients’ QOL before and after treatment; the score is inversely proportional to the patient’s QOL.
Statistical analysis:
Mean±Standard Error of the Mean (SEM) was used for the statistical description of measurement of data. Inter and intra-group comparisons (i.e., before and after treatment) were studied by independent sample t-test and paired t-test, respectively. Count data was expressed by the rate (percentage), and the comparison between two groups was made by the Chi-square (χ2) test. The collected experimental data were analyzed by Statistical Package of Social Sciences (SPSS) 22.0 and p<0.05 was considered to be statistically significant.
Results and Discussion
Baseline data of all the 120 COPD patients was studied. As shown in Table 1, the research group and the control group did not differ significantly in characteristics like sex, age, disease course, diabetes, hypertension, coronary artery disease, education level and other baseline data (p>0.05).
| Factors | Research group (n=62) | Control group (n=58) | χ2/t | p |
|---|---|---|---|---|
| Sex (male/female) | 38/24 | 32/26 | 0.462 | 0.497 |
| Age (y) | 59.63±10.30 | 57.05±10.39 | 1.365 | 0.175 |
| Disease course (y) | 3.35±0.89 | 3.48±0.75 | 0.862 | 0.390 |
| Diabetes mellitus (with/without) | 12/50 | 14/44 | 0.404 | 0.525 |
| Hypertension (with/without) | 33/29 | 28/30 | 0.294 | 0.588 |
| Coronary artery disease (yes/no) | 26/36 | 25/33 | 0.017 | 0.897 |
| Education level (below/above technical secondary school) | 35/27 | 38/20 | 1.034 | 0.309 |
Table 1: Baseline Data of COPD Patients
Curative effect of COPD patients, treated with two different therapies was studied. The research group was treated with SFP+TB and the results showed higher Objective Response Rate (ORR) than the control group treated with TB monotherapy (91.94 % vs. 75.86 %), with statistical significance (p<0.05) (Table 2).
| Factors | Research group (n=62) | Control group (n=58) | χ2 | p |
|---|---|---|---|---|
| Cure | 24 (38.71) | 14 (24.14) | - | - |
| Marked response | 26 (41.94) | 24 (41.38) | - | - |
| Response | 7 (11.29) | 6 (10.34) | - | - |
| Non-response | 5 (8.06) | 14 (24.14) | - | - |
| Overall response | 57 (91.94) | 44 (75.86) | 5.810 | 0.016 |
Table 2: Curative Effects of COPD Patients Treated with two different Therapies
Safety of COPD patients treated with two different therapies before and after was studied. According to the statistics the data analyzed that there were 6 patients (9.68 %) experiencing the above mentioned adverse reactions such as headache, constipation, palpitation and URTI, in the research group and 11 patients (18.97 %) in the control group. No significant inter-group difference (p>0.05) was observed as per the comparative analysis (Table 3).
| Factors | Research group (n=62) | Control group (n=58) | χ2 | p |
|---|---|---|---|---|
| Headache | 1 (1.61) | 2 (3.45) | ||
| Constipation | 2 (3.23) | 3 (5.17) | ||
| Palpitations | 1 (1.61) | 3 (5.17) | ||
| Upper respiratory tract infection | 2 (3.23) | 3 (5.17) | ||
| Total | 6 (9.68) | 11 (18.97) | 2.126 | 0.145 |
Table 3: Safety of COPD Patients Treated with two different Therapies
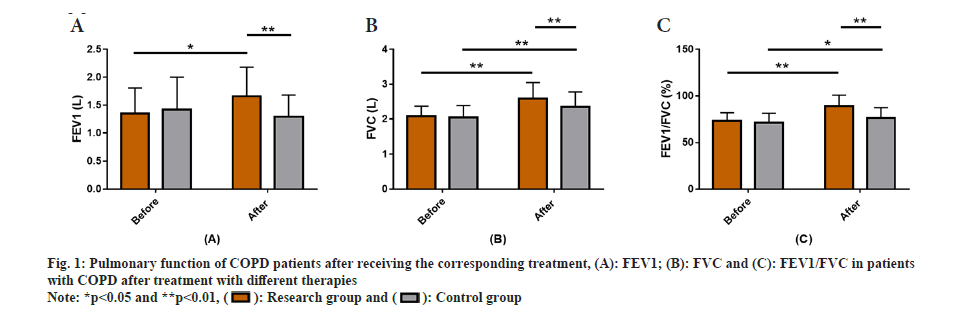
PF among all the COPD patients who were treated with two different therapies was observed. After receiving the corresponding treatment, FEV1, FVC and FEV1/FVC, the PF of among the COPD patients was detected and evaluated. The results showed no significant difference in pre-treatment while evaluating FEV1, FVC and FEV1/FVC between groups (p>0.05). However, after treatment FEV1, FVC and FEV1/FVC in the research group increased significantly, higher than those in the control group, with a statistical significance (p<0.05) (fig. 1).
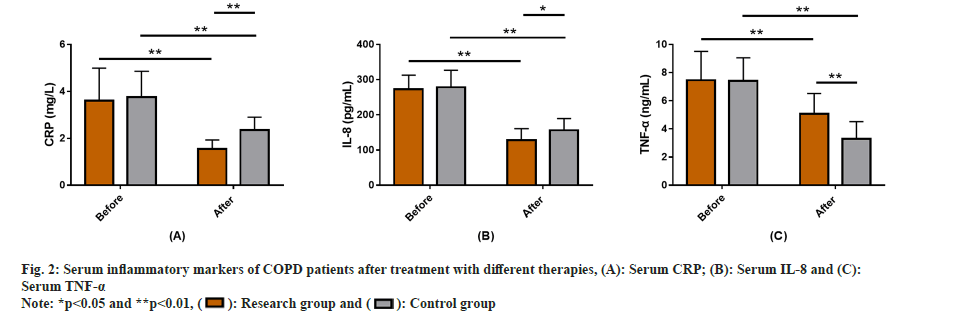
The serum inflammatory markers of all the COPD patients after treatment with different therapies were studied. Serum inflammatory markers included detection of CRP, IL-8 and TNF-α in both the groups after treatment with different therapies. The data showed no significant inter-group difference in the above indices before treatment (p>0.05). But the post-treatment serum inflammatory markers (CRP, IL-8 and TNF-α) reduced markedly in both groups, with more significant reductions in the research group (p<0.05) (fig. 2).
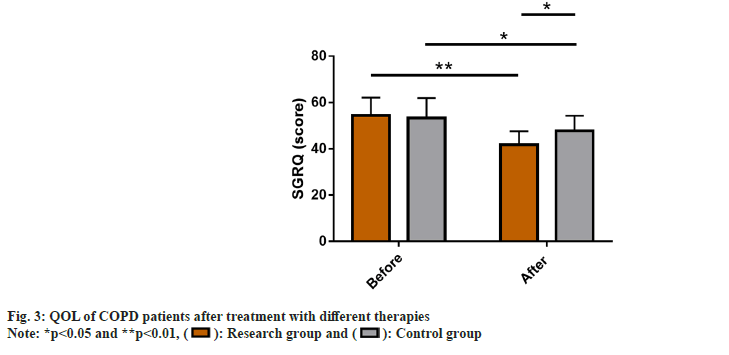
QOL of COPD patients after treatment with different therapies was observed in the two groups. The SGRQ score was used to evaluate the QOL of COPD patients after receiving different treatment modalities. The two groups did not differ much in pre-treatment SGRQ scores (p>0.05). An evident reduction was observed in the score of both the groups after therapy (p<0.05), with an even low score in the research group (p<0.05) (fig. 3).
COPD, which is related to airway or alveolar abnormalities, is one of the leading causes of death in developed countries with rising mortality[17]. The disease may also lead to systemic manifestations such as depression, anxiety, bone and muscle loss, and decreased exercise tolerance, adversely affecting the patients' physical and mental health with varying degrees of a normal life[18]. Therefore, in-depth analysis of novel COPD treatment strategies is of great significance for improving COPD management and mitigating the negative effects of the disease.
In our study, the research group was treated with SFP+TB and had a markedly higher ORR than the control group which was treated with TB alone (91.94 % vs. 75.86 %), suggesting a reliable therapeutic efficacy of SFP+TB therapy in COPD patients. In other words, combination of SFP with TB seems to have superior efficacy compared with TB monotherapy. SFP, as its name suggests, is composed of salmeterol and fluticasone propionate, has a therapeutic activity of up to 12 h, which not only helps to control the disease, but also has a lower frequency of administration than 2nd generation short-acting drugs[19]. In the latter, as an inhaled glucocorticoid, it often requires co-administration of long-acting drugs such as salmeterol, which is conducive in reducing the morbidity and mortality associated with the administration of salmeterol alone[20,21]. Therefore, the combination therapy of SFP and TB in this study can also be referred to as the triple therapy of salmeterol, fluticasone propionate, and TB. One study suggests that SFP has a certain influence on airway microflora in COPD patients, characterized by relatively less Alpha (α)- diversity and more variation in bacterial groups, which may help to partially explain its therapeutic mechanisms[22]. Furthermore, the number of adverse reactions such as headache, constipation, palpitation, and URTI was found to be similar in the two groups, suggesting that SFP+TB does not increase the risk of drug use and is well tolerated, similar to the research results of Miravitlles et al.[23]. In terms of PF, significantly higher FEV1, FVC, and FEV1/FVC were determined in the research group vs. control group, indicating that the combined action of the both is more advantageous to the improvement of PF in COPD patients.
TB and SFP are both long-acting bronchodilators for COPD maintenance treatment, and their combination is more effective than either of them, which is further demonstrated in this study[24,25]. Another study pointed out that SFP+TB would not have a significant impact on PF in patients by worsening COPD, suggesting that this therapy is tolerated in patients with exacerbations of COPD[26]. As far as serum inflammatory markers are concerned, CRP, IL-8, and TNF-α were found to be notably inhibited in the research group after treatment. It was lower when compared with the control group, demonstrating the ability of SFP+TB to inhibit serum inflammatory responses in COPD patients. These inflammatory markers are known to be closely associated with COPD exacerbations[27,28]. Finally, the investigation of patients’ QOL revealed markedly lower SGRQ scores in the research group that were lower compared with the control group after treatment, indicating that SFP+TB therapy can significantly enhance the QOL of COPD patients. As indicated by Hamid et al.[29], the effect of TB on PF, degree of disease resolution, and QOL of COPD patients would not be affected by the presence or absence of spacers in the delivery device.
Conclusively, SFP+TB outperformed TB monotherapy in the treatment of COPD, which can significantly improve PF, inhibit serum inflammatory markers, and exert a significant positive impact on the improvement of QOL without increasing the risk of medication, which is worth promotion clinically. Our research can provide a new medication option for COPD patients and novel insights in COPD management.
Conflict of interests:
The authors declared no conflict of interests.
References
- Segal LN, Martinez FJ. Chronic obstructive pulmonary disease subpopulations and phenotyping. J Allergy Clin Immunol 2018;141(6):1961-71.
[Crossref] [Google Scholar] [PubMed]
- Ritchie AI, Wedzicha JA. Definition, causes, pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin Chest Med 2020;41(3):421-38.
[Crossref] [Google Scholar] [PubMed]
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186(2):155-61.
[Crossref] [Google Scholar] [PubMed]
- Li J, Sun S, Tang R, Qiu H, Huang Q, Mason TG, et al. Major air pollutants and risk of COPD exacerbations: A systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2016;11(1):3079-91.
[Crossref] [Google Scholar] [PubMed]
- Lee YG, Lee PH, Choi SM, An MH, Jang AS. Effects of air pollutants on airway diseases. Int J Environ Res Public Health 2021;18(18):1-17.
[Crossref] [Google Scholar] [PubMed]
- Gabriel R, Figueiredo D, Jacome C, Cruz J, Marques A. Day-to-day living with severe chronic obstructive pulmonary disease: Towards a family-based approach to the illness impacts. Psychol Health 2014;29(8):967-83.
[Crossref] [Google Scholar] [PubMed]
- Lenferink A, Brusse-Keizer M, van der Valk PD, Frith PA, Zwerink M, Monninkhof EM, et al. Self-management interventions including action plans for exacerbations vs. usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;8(8):CD011682.
[Crossref] [Google Scholar] [PubMed]
- Wang T, Mao L, Wang J, Li P, Liu X, Wu W. Influencing factors and exercise intervention of cognitive impairment in elderly patients with chronic obstructive pulmonary disease. Clin Interv Aging 2020;15:557-66.
[Crossref] [Google Scholar] [PubMed]
- Hanania NA, O'Donnell DE. Activity-related dyspnea in chronic obstructive pulmonary disease: Physical and psychological consequences, unmet needs, and future directions. Int J Chron Obstruct Pulmon Dis 2019;14:1127-1138.
[Crossref] [Google Scholar] [PubMed]
- Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler vs. inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;8:1-109.
[Crossref] [Google Scholar] [PubMed]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356(8):775-89.
[Crossref] [Google Scholar] [PubMed]
- Steiropoulos P, Tryfon S, Kyriakopoulos C, Bartziokas K, Kostikas K. Evaluation of the clinical effectiveness of the salmeterol/fluticasone fixed-dose combination delivered via the elpenhaler® device in Greek patients with chronic obstructive pulmonary disease and comorbidities: The AEOLOS study. J Pers Med 2021;11(11):1159.
[Crossref] [Google Scholar] [PubMed]
- Zhou XJ, Qin Z, Lu J, Hong JG. Efficacy and safety of salmeterol/fluticasone compared with montelukast alone (or add-on therapy to fluticasone) in the treatment of bronchial asthma in children and adolescents: A systematic review and meta-analysis. Chin Med J 2021;134(24):2954-61.
[Crossref] [Google Scholar] [PubMed]
- Dhillon S. Tiotropium/olodaterol: A review in COPD. Drugs 2016;76(1):135-46.
[Crossref] [Google Scholar] [PubMed]
- Keating GM. Tiotropium Respimat® Soft mist™ inhaler: A review of its use in chronic obstructive pulmonary disease. Drugs 2014;74(15):1801-16.
[Crossref] [Google Scholar] [PubMed]
- Blair HA. Tiotropium/olodaterol: A review in COPD. Drugs 2019;79(9):997-1008.
[Crossref] [Google Scholar] [PubMed]
- Wouters EF, Wouters BB, Augustin IM, Houben-Wilke S, Vanfleteren LE, Franssen FM. Personalised pulmonary rehabilitation in COPD. Eur Respir Rev 2018;27(147):1-8.
[Crossref] [Google Scholar] [PubMed]
- Regan EA, Hersh CP, Castaldi PJ, deMeo DL, Silverman EK, Crapo JD, et al. Omics and the search for blood biomarkers in chronic obstructive pulmonary disease. Insights from COPDGene. Am J Respir Cell Mol Biol 2019;61(2):143-9.
[Crossref] [Google Scholar] [PubMed]
- Cazzola M, Page CP, Rogliani P, Matera MG. β2-agonist therapy in lung disease. Am J Respir Crit Care Med 2013;187(7):690-6.
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with salmeterol and inhaled steroids for chronic asthma: Serious adverse events. Cochrane Database Syst Rev 2009;3:1-95.
[Crossref] [Google Scholar] [PubMed]
- O’Shea O, Stovold E, Cates CJ. Regular treatment with formoterol for chronic asthma: Serious adverse events. Cochrane Database Syst Rev 2012;4:1-88.
[Crossref] [Google Scholar] [PubMed]
- Filho FSL, Takiguchi H, Akata K, Ra SW, Moon JY, Kim HK, et al. Effects of inhaled corticosteroid/long-acting β2-agonist combination on the airway microbiome of patients with chronic obstructive pulmonary disease: A randomized controlled clinical trial (DISARM). Am J Respir Crit Care Med 2021;204(10):1143-52.
[Crossref] [Google Scholar] [PubMed]
- Miravitlles M, Urrutia G, Mathioudakis AG, Ancochea J. Efficacy and safety of tiotropium and olodaterol in COPD: A systematic review and meta-analysis. Respir Res 2017;18(1):196.
[Crossref] [Google Scholar] [PubMed]
- Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 vs. single bronchodilator therapy: The SHINE study. Eur Respir J 2013;42(6):1484-94.
[Crossref] [Google Scholar] [PubMed]
- Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016;149(5):1181-96.
[Crossref] [Google Scholar] [PubMed]
- Chapman KR, Hurst JR, Frent SM, Larbig M, Fogel R, Guerin T, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): A randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med 2018;198(3):329-39.
[Crossref] [Google Scholar] [PubMed]
- Fermont JM, Masconi KL, Jensen MT, Ferrari R, di Lorenzo VA, Marott JM, et al. Biomarkers and clinical outcomes in COPD: A systematic review and meta-analysis. Thorax 2019;74(5):439-46.
[Crossref] [Google Scholar] [PubMed]
- Feng Y, Liu E. Detection of respiratory viruses and expression of inflammatory cytokines in patients with acute exacerbation chronic obstructive pulmonary disease in Mongolia China. Braz J Biol 2021;82:1-8.
[Crossref] [Google Scholar] [PubMed]
- Hamid MF, Munusamy H, Mohamad MF, Ban L. Comparison of clinical efficacy and satisfaction of tiotropium via Respimat® administration with and without a spacer in patient with chronic obstructive pulmonary disease: A single centre experience. Med J Malaysia 2022;77(4):481-7.
[Google Scholar] [PubMed]