- *Corresponding Author:
- Changxi Zhang
Department of Thoracic Surgery, The Chonggang General Hospital Affiliated to Chongqing University of Posts and Telecommunications, Dadukou, Chongqing 400080, China
E-mail: 13883855846@126.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “347-352” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this research was to evaluate the effects of tislelizumab in conjunction with albuminbound paclitaxel and carboplatin on the gut microbiota, systemic inflammation index, and prognostic nutritional index in individuals with advanced non-small cell lung cancer. Over a period spanning from October 2021 to September 2023, a review of 150 patients undergoing treatment for advanced non-small cell lung cancer was carried out at our hospital. These individuals were randomly allocated into two groups, with 75 individuals in each. Albumin-bound paclitaxel and carboplatin were administered to the control group, whereas the observation group received an additional course of tislelizumab treatment. The treatment procedure comprised 21 d cycles, with a total of 3 cycles administered. The research encompassed the assessment of the objective response rate, disease control rate, serum tumor markers, systemic inflammation index, prognostic nutritional index, gut microbiota composition, and safety profile. The objective response rate was substantially higher in the observation group at 46.67 % as opposed to the control group’s 28.00 %, with a disease control rate of 77.33 % in the observation group in relative to 61.33 % in the control group, exhibiting noteworthy differences (p<0.05). Additionally, levels of cytokeratin 19 fragment, carcinoembryonic antigen, and carbohydrate antigen 125 in the observation group markedly decreased in comparison to the control group following treatment (p<0.05). Remarkably higher levels of bifidobacteria and lactobacilli, and notably lower levels of clostridium perfringens were found in the gut microbiota of the observation group relative to the control group (p<0.05). The observation group also displayed lower systemic inflammation index and elevated prognostic nutritional index in comparison to the control group (p<0.05). No notable distinction in adverse reaction incidence was identified between groups (p>0.05). The incorporation of tislelizumab alongside albumin-bound paclitaxel and carboplatin presented notable clinical effectiveness in advanced non-small cell lung cancer patients, resulting in reductions in tumor marker levels and advantageous modifications in gut microbiota composition, systemic inflammation index, and prognostic nutritional index. Furthermore, the treatment protocol exhibited excellent tolerability and a positive safety profile.
Keywords
Tislelizumab, albumin-bound paclitaxel, carboplatin, advanced non-small cell lung cancer
The majority, around 80 %-85 % of lung cancer cases are attributed to Non-Small Cell Lung Cancer (NSCLC) [1]. Frequently, NSCLC does not exhibit noticeable symptoms during the initial stages, and due to the relatively slow rate of cancer cell division, patients are often diagnosed during advanced stages, impacting the opportunity for optimal surgical intervention and leading to chemotherapy being the favored treatment option[2,3]. Carboplatin is established as the primary chemotherapeutic agent for managing NSCLC, while paclitaxel, classified as a taxane medication, is widely applied in cancer chemotherapy[4-6]. Additionally, albumin-bound paclitaxel, a semi-synthetic taxane drug, has exhibited favorable effectiveness in certain solid tumors like breast cancer and liver cancer; however, chemotherapy may evoke diverse adverse reactions[7,8]. Demonstrating positive antitumor activity in advanced NSCLC, tislelizumab injection is a humanized Immunoglobulin (IgG)-4 monoclonal antibody designed to target programmed cell death receptor 1[9-11].
The gut microbiota, counted among the human body’s largest microbial communities, plays a crucial part in immune responses, nutrient absorption, and drug metabolism, thus prompting greater focus on its impact on lung cancer treatment[12,13]. In addition, Systemic Inflammation Index (SII) and the Prognostic Nutritional Index (PNI) act as markers that mirror the comprehensive inflammatory condition and nutritional well-being of patients-a valuable contribution to the assessment of their immune function and overall health status[14,15].
This study seeks to examine the impact of combining tislelizumab injection with albumin-bound paclitaxel and carboplatin on the gut microbiota, SII, and PNI in individuals with advanced NSCLC. The objective is to lay the groundwork for the customization of treatment regimens and improving the efficacy of treatment and the patient’s life quality. By delving into the interrelation between these indicators, it is hoped that new perspectives will be provided for microbial and immunological monitoring in lung cancer treatment, offering theoretical support for the optimization of future lung cancer treatment strategies.
Materials and Methods
Clinical data:
A total of 150 individuals with advanced NSCLC who were undergoing treatment at our hospital between October 2021 and September 2023 were retrospectively identified. Subsequently, these patients were allocated into a control group and an observation group (75 in each). Within the control group, there were 49 male and 26 female patients, aged between 60 y and 85 y (70.41±8.57) y. Squamous cell carcinoma was observed in 24 cases, while adenocarcinoma and adenosquamous carcinoma were present in 39 and 12 cases, respectively, within the distribution of pathologic types. 25 cases were classified as stage IIIA, 43 as stage IIIB, and 7 as stage IV in the Tumour, Node, Metastasis (TNM) staging. Within the observation group, the demographic consisted of 47 male and 28 female individuals, aged between 61 y and 84 y (69.83±8.63) y. Of the cases, 26 were identified as squamous cell carcinoma, 38 as adenocarcinoma, and 11 as adenosquamous carcinoma in the distribution of pathologic types. Moreover, 24 cases were classified as stage IIIA, 45 as stage IIIB, and 6 as stage IV in the TNM staging. Demonstrating comparability, there were no detectable discrepancies in age, gender, or pathologic type between groups (p>0.05). Furthermore, this research obtained ethical approval from the Hospital Medical Ethics Committee.
Inclusion criteria: NSCLC diagnosis criteria in accordance with “Chinese Expert Consensus on Diagnosis and Treatment of Advanced Primary Lung Cancer (2016 Edition)”[16], confirmed by pathological examination with atleast one measurable tumor lesion; all patients had squamous cell carcinoma and negative driver genes; informed consent signed by patients or their family members; Karnofsky Performance Status (KPS) score ≥60, life expectancy >3 mo and clinical TNM staging IIIB~IV.
Exclusion criteria: Lung adenocarcinoma, large cell carcinoma; severe organ dysfunction; concurrent autoimmune diseases; pregnancy or lactation; coagulation disorders; previous participation in another treatment plan; concurrent other malignancies; history of mental illness and allergy to the drugs used in this study or contraindications[17].
Methods:
The control group was administered with albuminbound paclitaxel injection (100 mg) obtained from the Celgene corporation, United States of America (USA), with registration number H20130650, and carboplatin injection (5 ml:50 mg) produced by Qilu Pharmaceutical Co., Ltd., approved by the China Food and Drug Administration (CFDA) under the number H20227082. Given via a 30 min intravenous infusion on d 1, 8, and 15 of a 21 d cycle, the dosage of albuminbound paclitaxel was 100 mg/m2. For carboplatin, it was diluted in 5 % glucose solution at a concentration of 10 mg/ml and given as an intravenous infusion of 200~400 mg/m2 over 250-500 ml of 5 % glucose injection on d 1 of a 21 d cycle. The observation group was given tislelizumab injection (100 mg (10 ml)/ bottle) from BeiGene Biopharmaceutical Co., Ltd., approved by CFDA under the number S20190045. This was administered in conjunction with the treatment provided to the control group, involving an intravenous infusion of 200 mg on d 1 within a 21 d cycle. Both groups underwent 3 cycles of treatment.
Efficacy evaluation criteria:
Achieving Complete Remission (CR) involved the disappearance of lesions that persisted for over 4 w, whereas Partial Remission (PR) was indicated by a combined reduction in the total measurable lesion diameter of at least 30 %, sustained for over 4 w. The definition of Disease Progression (PD) encompassed an increase of 20 % or more in the overall measurable lesion diameter, or the development of new lesions. Meanwhile, Stable Disease (SD) was implicated when cases fell between PR and PD. Contained within the Objective Response Rate (ORR) were the combined rates of CR and PR, while the Disease Control Rate (DCR) encompassed the cumulative rates of CR, PR, and SD.
Observation indicators:
Serum tumor markers: In SII and PNI testing, both pre-treatment and post-treatment, 5 ml of peripheral blood samples were gathered from each individual. The Sysmex XN-2800 automated blood cell analyzer (HISCL, Japan) was employed to calculate the neutrophil count, lymphocyte count, and platelet count. Additionally, the KEA-TR100 automated biochemical analyzer (Shangyikang, Wuhan, China) was utilized to assess serum albumin levels. The calculation of PNI involved the addition of the serum albumin (g/l) to five times the lymphocyte count (109/l), and the SII was obtained by multiplying the platelet count by the neutrophil count and then dividing by the lymphocyte count (109/l). The concentrations of serum Carcinoembryonic Antigen (CEA), Cytokeratin 19 Fragment (CYFRA21-1), and Carbohydrate Antigen 125 (CA125) were determined utilizing Enzyme- Linked Immunosorbent Assay (ELISA). Our supply for the CEA kit was sourced from Wuhan Fei’en Biotechnology Co., Ltd., the CYFRA21-1 kit from Shanghai Fuyu Biotechnology Co., Ltd., and the CA125 kit from Wuhan Yipu Biotechnology Co., Ltd
Gut microbiota testing: Stool samples (4-6) g were freshly collected before and after treatment and frozen at -80°. Upon dissolving in physiological saline, the samples were plated on agar culture medium. Anaerobic bacteria cultures were incubated at 37° for 48 h, and aerobic bacteria cultures at 37° for 24 h. Quantification of Bifidobacterium, Lactobacillus, and Enterococcus in each gram of fecal matter was carried out, with the results expressed in log colony-forming units per gram (log CFU/g).
Safety assessment: The treatment period saw the occurrence of adverse reactions among all patients, such as decreased white blood cell count, gastrointestinal disturbances, rash, fatigue, reduced neutrophil count, lowered platelet levels, hypothyroidism, and elevated alanine aminotransferase level.
Statistical methods:
The analysis compared the measurement data between the two groups utilizing the Statistical Package for the Social Sciences (SPSS) 25.0 software, presented as mean±standard deviation, and employed the Chisquare (χ2) test for analyzing the count data, with notable significance defined at p<0.05.
Results and Discussion
Both the ORR and DCR of the observation group, at 46.67 % and 77.33 % respectively, were remarkably higher than those of the control group, which had an ORR of 28.00 % and DCR of 61.33 % (p<0.05) (Table 1).
| Group (n=75) | CR | PR | SD | PD | ORR | DCR |
|---|---|---|---|---|---|---|
| Observation | 0 (0.00) | 35 (46.67) | 23 (30.67) | 17 (22.67) | 35 (46.67) | 58 (77.33) |
| Control | 0 (0.00) | 21 (28.00) | 25 (33.33) | 29 (38.67) | 21 (28.00) | 46 (61.33) |
| χ2 | 5.585 | 4.515 | ||||
| p | 0.018 | 0.034 |
Table 1: Orr and Dcr
At the outset of treatment, there were no notable variations in CYFRA21-1, CEA, and CA125 levels between groups (p>0.05). Following treatment, the observation group revealed decreased levels of CYFRA21-1, CEA, and CA125 compared to the control group (p<0.05). After 2 treatment cycles, the CYFRA21-1, CEA, and CA125 levels decreased in both groups (p<0.05) (Table 2).
| Group (n=75) | CYFRA21-1 | CEA | CA125 | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation | 32.92±4.91 | 13.20±4.06* | 12.30±3.50 | 4.43±1.35* | 191.96±32.08 | 93.53±25.12* |
| Control | 32.00±4.64 | 22.21±5.16* | 13.69±3.24 | 7.47±1.56* | 191.42±34.11 | 131.07±29.03* |
| T | -1.173 | 11.880 | 1.413 | 7.384 | -0.099 | 8.399 |
| P | 0.243 | 0.000 | 0.16 | 0.000 | 0.921 | 0.000 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 2: Hemorheological Level (Mpa•S)
No marked differences were observed in Bifidobacterium, Lactobacillus, and Enterococcus levels between groups prior to treatment (p>0.05). However, following treatment, the observation group displayed higher levels of Bifidobacterium and Lactobacillus, along with lower levels of Enterococcus (p<0.05). Following 2 treatment cycles, the levels of Bifidobacterium and Lactobacillus decreased, while the level of Enterococcus elevated in both groups (p<0.05) (Table 3).
| Group (n=75) | Bifidobacterium | Lactobacillus | Enterococcus | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Observation | 6.70±0.95 | 4.32±0.98* | 9.28±1.42 | 7.28±0.99* | 5.31±1.28 | 6.48±1.32* |
| Control | 6.79±0.89 | 3.84±0.96* | 8.77±1.38 | 4.60±0.98* | 5.19±1.21 | 6.13±1.25* |
| T | 0.620 | -3.081 | -1.233 | -16.645 | -0.598 | -2.633 |
| P | 0.536 | 0.002 | 0.220 | 0.000 | 0.551 | 0.009 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 3: Intestinal Flora (Lg Cfu/G)
Prior to treatment, no noteworthy disparities were present in the SII and PNI levels between groups (p>0.05). Post-treatment, the observation group demonstrated a lower SII compared to the control group, while exhibiting a higher PNI (p<0.05). After 2 treatment cycles, both groups experienced a decrease in SII and an increase in PNI (p<0.05) (fig. 1).
The overall incidence of adverse reactions of the observation group amounted to 16.00 %, in contrast to 14.67 % in the control group. Notably, no remarkable differences in the incidence of adverse reactions between groups were identified (p>0.05) (Table 4).
| Group (n=75) | Neutropenia | Rash | Thrombopenia | Gastrointestinal reaction | Fatigued | Overall incidence |
|---|---|---|---|---|---|---|
| Observation | 3 (4.00) | 1 (1.33) | 3 (4.00) | 2 (2.67) | 3 (4.00) | 12 (16.00) |
| Control | 3 (4.00) | 2 (2.67) | 1 (1.33) | 3 (4.00) | 2 (2.67) | 11 (14.67) |
| χ2 | 0.051 | |||||
| P | 0.821 |
Table 4: Adverse Reactions N (%)
This study’s results reveal that tislelizumab injection, when combined with albumin-bound paclitaxel and carboplatin, demonstrates superior clinical efficacy in individuals with NSCLC in comparison to the conventional treatment using paclitaxel and carboplatin.
Initially, we noted that the observation group demonstrated a significantly elevated ORR of 46.67 % and DCR of 77.33 %, both exceeding the control group’s respective ORR of 28.00 % and DCR of 61.33 %. These findings indicate notable advantages in achieving disease remission and control with the combined administration of tislelizumab injection, albuminbound paclitaxel, and carboplatin. This integrated treatment strategy displays potential advantages in clinical efficacy by hindering tumor cell proliferation, differentiation, and invasion through diverse pathways, ultimately enhancing overall clinical effectiveness.
CYFRA21-1, widely utilized as a tumor marker, is linked to lung cancer cell metastasis and proliferation[18,19]. Furthermore, elevated levels of CEA and CA125, also commonly used tumor markers in lung cancer, indicate heightened tumor cell activity[20]. In this study, we noted that post-treatment, the serum tumor marker levels of CYFRA21-1, CEA, and CA125 were notably decreased in the observation group. After 2 treatment cycles, the levels of these markers decreased in both groups. These observations indicate that tislelizumab injection may have a certain inhibitory effect on tumor growth and metastasis.
Furthermore, our assessment of gut microbiota profiles revealed that the observation group had increased levels of Bifidobacterium and Lactobacillus, along with diminished levels of Enterococcus compared to the control group. Subsequently, after 2 treatment cycles, we witnessed a reduction in the levels of Bifidobacterium and Lactobacillus, while the level of Enterococcus increased in both groups. This suggests that the administration of tislelizumab injection could impact the gut microbiota to generate a specific therapeutic response, and this may correlate with treatment efficacy and the immune status of the patients.
In conclusion, we identified a considerable decrease in the SII and a substantial increase in the PNI within the observation group in relative to the control group. After 2 treatment cycles, both the SII and PNI decreased, indicating that the combined use of tislelizumab injection, albumin-bound paclitaxel, and carboplatin offers advantages in antitumor effects and improves the inflammatory and nutritional statuses of patients. This could be attributed to the capacity of tislelizumab injection to not only provide antitumor effects but also amplify the therapeutic efficacy of albumin-bound paclitaxel and carboplatin, synergistically inhibiting tumor advancement and subsequently, ameliorating SII and PNI.
Of significance, the overall incidence of adverse reactions in the observation group paralleled that in the control group, without any noteworthy disparity. This implies that the treatment plan involving tislelizumab injection combined with albumin-bound paclitaxel and carboplatin does not disproportionately heighten the risk of adverse reactions when contrasted with the use of paclitaxel and carboplatin alone.
To summarize, the outcomes of this study imply that the utilization of tislelizumab injection in conjunction with albumin-bound paclitaxel and carboplatin provides enhanced clinical effectiveness in individuals with advanced NSCLC, surpassing the single use of carboplatin and paclitaxel. Furthermore, this combination treatment could potentially bring about positive effects on biological parameters, encompassing serum tumor markers, gut microbiota, SII, and PNI. However, additional research is necessary to substantiate these findings and delve into their underlying mechanisms thoroughly.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhou G, Pu Y, Zhao K, Chen Y, Zhang G. Heat shock proteins in non-small-cell lung cancer-functional mechanism. Front Biosci Landmark 2023;28(3):56.
[Crossref] [Google Scholar] [PubMed]
- Glatzer M, Elicin O, Ramella S, Nestle U, Putora PM. Radio (chemo) therapy in locally advanced nonsmall cell lung cancer. Eur Respir Rev 2016;25(139):65-70.
[Crossref] [Google Scholar] [PubMed]
- Borghetti P, Branz J, Volpi G, Pancera S, Buraschi R, Bianchi LN, et al. Home-based pulmonary rehabilitation in patients undergoing (chemo) radiation therapy for unresectable lung cancer: A prospective explorative study. Radiol Med 2022;127(12):1322-32.
[Crossref] [Google Scholar] [PubMed]
- Blair HA, Deeks ED. Albumin-bound paclitaxel: A review in non-small cell lung cancer. Drugs 2015;75:2017-24.
[Crossref] [Google Scholar] [PubMed]
- Wu YL, Saijo N, Thongprasert S, Yang JH, Han B, Margono B, et al. Efficacy according to blind independent central review: Post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib vs. carboplatin/paclitaxel in Asian patients with EGFR mutation-positive advanced NSCLC. Lung Cancer 2017;104:119-25.
[Crossref] [Google Scholar] [PubMed]
- Thomas QD, Chaabouni M, Al herk A, Lefevbre C, Cavaillon S, Sinoquet L, et al. Exploring the efficacy of pembrolizumab in combination with carboplatin and weekly paclitaxel for frail patients with advanced non-small-cell lung cancer: A key investigative study. Cancers 2024;16(5):992.
[Crossref] [Google Scholar] [PubMed]
- Lorenz E, Weitz A, Reinstaller T, Hass P, Croner RS, Benedix F. Neoadjuvant radiochemotherapy with cisplatin/5-flourouracil or carboplatin/paclitaxel in patients with resectable cancer of the esophagus and the gastroesophageal junction-comparison of postoperative mortality and complications, toxicity, and pathological tumor response. Langenbecks Arch Surg 2023;408(1):429.
[Crossref] [Google Scholar] [PubMed]
- Senguttuvan RN, Wei C, Raoof M, Dellinger TH, Wang EW. Complete pathologic response to PARP inhibitor Olaparib in a patient with stage IVB recurrent endometrioid endometrial adenocarcinoma. J Clin Med 2023;12(11):3839.
[Crossref] [Google Scholar] [PubMed]
- Jonna S, Subramaniam DS. Molecular diagnostics and targeted therapies in Non-Small Cell Lung Cancer (NSCLC): An update. Discov Med 2019;27(148):167-70.
[Google Scholar] [PubMed]
- Zhou C, Huang D, Fan Y, Yu X, Liu Y, Shu Y, et al. Tislelizumab vs. docetaxel in patients with previously treated advanced NSCLC (RATIONALE-303): A phase 3, open-label, randomized controlled trial. J Thorac Oncol 2023;18(1):93-105.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs. chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol 2021;7(5):709-17.
[Crossref] [Google Scholar] [PubMed]
- Song P, Yang D, Wang H, Cui X, Si X, Zhang X, et al. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac Cancer 2020;11(6):1621-32.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Liu D, Zhou H, Liu X, Li X, Cheng Y, et al. Intestinal flora characteristics of advanced non-small cell lung cancer in China and their role in chemotherapy based on metagenomics: A prospective exploratory cohort study. Thorac Cancer 2021;12(24):3293-303.
[Crossref] [Google Scholar] [PubMed]
- Fang Q, Yu J, Li W, Luo J, Deng Q, Chen B, et al. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin Exp Pharmacol Physiol 2023;50(2):178-90.
[Crossref] [Google Scholar] [PubMed]
- Zheng F, Meng Q, Zhang L, Chen J, Zhao L, Zhou Z, et al. Prognostic roles of hematological indicators for the efficacy and prognosis of immune checkpoint inhibitors in patients with advanced tumors: A retrospective cohort study. World J Surg Oncol 2023;21(1):198.
[Crossref] [Google Scholar] [PubMed]
- Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang C, et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Zhongguo Fei Ai Za Zhi 2016;19(1):1-5.
[Google Scholar] [PubMed]
- McNair KM, Zeitlin D, Slivka AM, Lequerica AH, Stubblefield MD. Translation of Karnofsky Performance Status (KPS) for use in inpatient cancer rehabilitation. PM R 2023;15(1):65-8.
[Crossref] [Google Scholar] [PubMed]
- Fu L, Wang R, Yin L, Shang X, Zhang R, Zhang P. CYFRA21-1 tests in the diagnosis of non-small cell lung cancer: A meta-analysis. Int J Biol Markers 2019;34(3):251-61.
[Crossref] [Google Scholar] [PubMed]
- Dal Bello MG, Filiberti RA, Alama A, Orengo AM, Mussap M, Coco S, et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med 2019;17(1):74.
[Crossref] [Google Scholar] [PubMed]
- Clevers MR, Kastelijn EA, Peters BJ, Kelder H, Schramel FM. Evaluation of serum biomarker CEA and CA-125 as immunotherapy response predictors in metastatic non-small cell lung cancer. Anticancer Res 2021;41(2):869-76.
[Crossref] [Google Scholar] [PubMed]
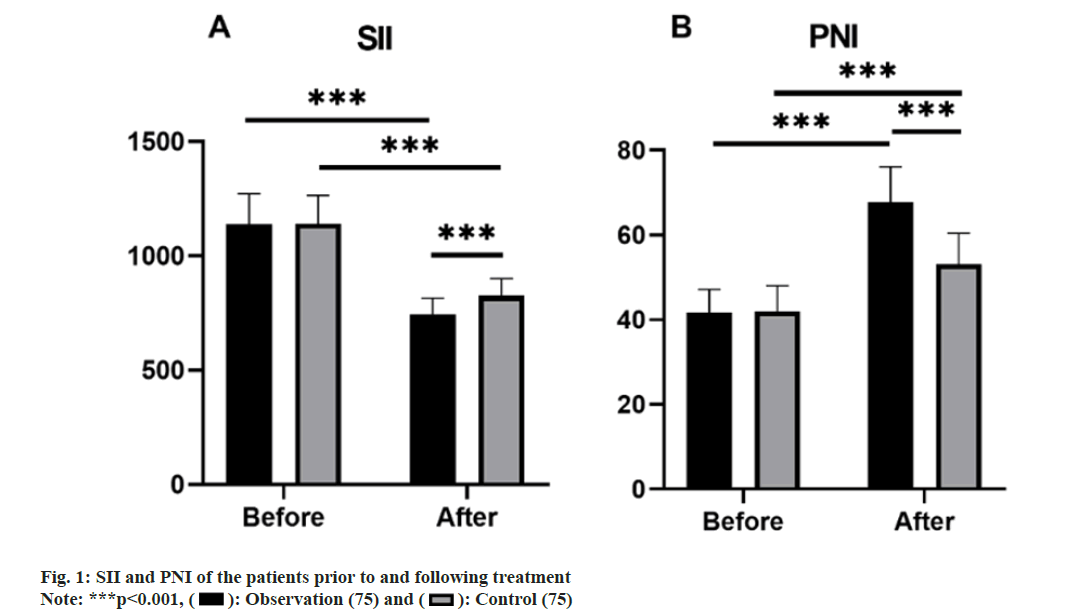
 ): Observation (75) and (
): Observation (75) and ( ): Control (75)
): Control (75)



