- *Corresponding Author:
- Yang Cao
Department of Anesthesiology, Ningbo Fourth Hospital, Ningbo, Zhejiang 315470, China
E-mail: 846576613@qq.com
| Date of Received | 30 October 2021 |
| Date of Revision | 14 September 2022 |
| Date of Acceptance | 20 July 2023 |
| Indian J Pharm Sci 2023;85(4):1077-1084 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of propofol pretreatment on microglial pyroptosis in the ischemic penumbra of rats subjected to cerebral ischemia-reperfusion. 90 rats were randomly divided into Sham group, model group, nod-like receptor protein 3 inhibitor group, propofol low dose group and high dose group. Pathological changes in the hippocampal region of the ischemic penumbra of brain tissue were observed by hematoxylin and eosin staining; interleukin-1 beta and interleukin-18 levels were detected in the ischemic penumbra of cerebral tissue by enzyme-linked immunosorbent assay; immunofluorescence double staining was performed to detect the immunopositive co-expression of nod-like receptor protein 3, aspartate specific caspase 1 and ionized calcium adaptor protein 1 in the ischemic penumbra of cerebral tissue. Compared with the model group, the nod-like receptor protein 3 inhibitor group, propofol low dose group and propofol high dose group groups also had significantly lower neurological deficit scores, cerebral infarction volumes, and interleukin-1 beta and interleukin-18 levels, ionized calcium adaptor protein 1/nod-like receptor protein 3, ionized calcium adaptor protein 1/caspase 1 immunopositive co-expression, nod-like receptor protein 3, pro-caspase 1, P10, P20 and interleukin-1 beta protein expressions in the ischemic penumbra were significantly reduced (p<0.05), the pathological damage was improved to various degrees, and the number of nerve cells was increased. The neuroprotective effect of propofol pretreatment on cerebral ischemia-reperfusion injury may be related to the inhibition of microglial pyroptosis mediated by nod-like receptor protein 3 inflammasome.
Keywords
Propofol, cerebral ischemia-reperfusion, pyroptosis, microglia, nod-like receptor protein 3
Stroke is characterized by high morbidity, mortality and disability, resulting in a heavy burden on patients, families and society. There are more than 11 million stroke patients in China, with 2.5 million new stroke cases annually, of which approximately 78 % are ischemic strokes[1,2]. Although progress has been made in terms of the pathological mechanism of stroke, an effective translation of this information to clinical applications has not been successful. Therefore, new therapeutic strategies still need to be developed.
Cerebral Ischemia Reperfusion (CIR) injury is a major pathophysiological mechanism of ischemic stroke[3]. The rapid decrease of blood flow in the early stage will lead to energy depletion and energy synthesis impairment, resulting in neuronal cell membrane depolarization as well as imbalance of intracellular and external ion homeostasis, which rapidly triggers a post ischemic cascade, such as excitotoxicity, oxidative stress, inflammation, edema and apoptosis[4]. The ischemic penumbra is a dysfunctional but not yet dead tissue surrounding the infarct due to ischemic injury, and timely improvement of hypoperfusion can restore normal, and therefore, protecting the ischemic penumbra is an important strategy for the treatment of CIR injury. Recently, studies have found that microglial pyroptosis mediated inflammation plays an important role in CIR injury[5,6]; while the Nod-Like Receptor
Protein 3 (NLRP3) inflammasomes, which is widely expressed in glia and peripheral immune cells in the brain and is activated in cerebral ischemia, is a key mediator of the post CIR inflammatory response by activating caspase-1, triggering the release of Interleukin (IL)-1 Beta (β) and IL-18, involved in the initiation and amplification of the inflammatory response[7]; targeting the NLRP3 inflammasomes and microglia to suppress excessive inflammatory responses may therefore be a novel therapeutic strategy for cerebral ischemia. Shen et al.[8] showed that reducing microglial activation in the ischemic penumbra could exert cerebroprotective effects in CIR rats. Propofol is a widely used general anesthetic in the clinic, and recent studies have found that, in addition to its anesthetic effects, propofol has antioxidant, anti-inflammatory and neuroprotective properties[9-11], which can attenuate renal ischemiareperfusion injury by down-regulating NLRP3 expression[12]; and propofol can inhibit the increase of NLRP1 and NLRP3 inflammasomes expression induced by Oxygen Glucose Deprivation (OGD) and alleviate neuroinflammatory injury[13]. Wen et al.[14] found that propofol post-treatment could exert neuroprotective effects in rats with cerebral ischemia by inhibiting the NLRP3/caspase 1 pathway; but none of the previous reports described the effect of propofol pretreatment on NLRP3 mediated microglial pyroptosis after CIR injury. Therefore, this study aimed to investigate the effects of propofol pretreatment on microglial pyroptosis and NLRP3 signaling pathway in the ischemic penumbra after CIR, to reveal the function of propofol mediated neuroprotective mechanisms in CIR injury.
Materials and Methods
Animals:
Ninety Sprague–Dawley (SD) rats (Specific Pathogen Free (SPF) grade), male and body mass (250-300) g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (License SCXK (J) 2019-0009). Rats were housed in the environment of temperature (20°±2°) and humidity (45 %-60 %) and maintained on a 12 h light/12 h dark cycle.
Reagents and instruments:
Propofol injection (Sichuan Guorui Pharmaceutical Co., Ltd., 20 ml: 200 mg, SFDA Approval No: H20030115); NLRP3 inhibitor group (MCC950, MedChemExpress LLC, HY-12815A); suture plug (Beijing Cinontech Co., Ltd.); 2,3,5-Triphenyltetrazolium chloride (TTC) staining solution (G3005) was purchased from Beijing Solarbio Co., Ltd.; Hematoxylin and Eosin (HE) staining kit (C0105) was purchased from Beyotime Biotech Inc., Shanghai.; IL-1β (R0012c), IL- 18 (R0567c) Enzyme-Linked Immunosorbent Assay (ELISA) kits were purchased from Wuhan Elabscience Biotechnology Co., Ltd.; goat anti- Ionized Calcium Adaptor Protein 1 (IBA1) (011- 27991) was purchased from Wako, Japan; rabbit anti- NLRP3 (ab263899), Alexa Fluor 488 labeled donkey anti-goat Immunoglobulin G (IgG) H&L (ab150129, green fluorescence), Alexa Fluor® 647 labeled goat anti-rabbit IgG H&L (ab150079, red fluorescence), pro caspase-1 (ab207802), β-actin (ab8227), IL-1β (ab200478), IgG secondary antibodies (goat antirabbit (ab205718), goat anti-mouse (ab205719)) were purchased from Abcam, UK; mouse primary antibodies cleaved caspase-1 P10, cleaved caspase-1 P20 were purchased from Santa Cruz, United States of America (USA), Cat No.: sc-56036, sc-398715. Microplate reader (iMark680, Bio-Rad, USA); light microscope (BX51, Olympus Corporation, Japan).
Grouping and modeling:
The rats were divided into sham operated group, model group, NLRP3 inhibitor group (MCC950, 10 mg/kg), propofol Low group (propofol-L, 10 mg/kg), and propofol High dose group (propofol-H, 20 mg/ kg) (n=18)[14]. NLRP3 inhibitor group and propofol group received the corresponding doses of MCC950 and propofol via tail vein injection 30 min before Middle Cerebral Artery Occlusion (MCAO); the model group was injected with an equal volume of normal saline. The MCAO model was constructed in all rats except the sham operated group[15]; rats were anesthetized by intraperitoneal injection of 40 mg/kg sodium pentobarbital, a small port was cut at the median of the neck to free the right Common Carotid Artery (CCA), External Carotid Artery (ECA) and Internal Carotid Artery (ICA), the ECA root was ligated with a suture and the proximal end of CCA was ligated; clamping the ICA, a wire plug (0.38 mm in diameter) was slowly inserted into the ICA from the CCA to occlude the middle cerebral artery and stopped (about 18-20 mm) when feeling slight resistance, the wire plug was fixed; the wire plugs were pulled out about 15 mm after 2 h to form reperfusion. Sham operated group was operated as above except that the suture plug was not inserted.
After the rats were awake, the neurobehaviors of the rats after modeling were scored according to the Zea-Longa scoring method, and rats with scores of 1-3 were taken for experiments (n=18 in each group, and rats with death and unsuccessful modeling were randomly supplemented). Sample collection was performed after 24 h of reperfusion.
Sampling and index detection:
Neurological deficit scoring: Neurological deficit scoring was performed according to the Zea- Longa scoring method after 24 h of reperfusion. Neurological function was normal with no deficit scored as 0 point; inability of the right forepaw to fully extend scored as 1 point; turning the circle to the contralateral side counted 2 points; tilting to left side scored 3 points; no spontaneous walking, loss of consciousness, or death scored 4 points. Rats with scores of 0 and 4, and subarachnoid hemorrhage were excluded. All assessments were performed by investigators blinded to treatment group.
Cerebral infarction volume: After completion of neurological deficit scoring, six rats from each group were randomly selected, and the brains were quickly removed after sacrifice. Brains were rapidly frozen at -20° and cut into 2 mm thick coronal sections. Staining was performed with 1 % TTC for 20 min at 37°, followed by fixation in 4 % paraformaldehyde for 2 h. Normal brain areas were stained deep red and infarction areas were stained white. Coronal sections were photographed and the total infarction volume was calculated with Image Pro Plus 6.0 software. To correct for edema and atrophy, the cerebral infarction volume was calculated as:
Infarct volume (%)=(Contralateral hemisphere areaipsilateral hemisphere non-infarct area)/contralateral hemisphere area×100 %
ELISA detection of IL-1 β and IL-18 levels: Six rats were randomly selected from each group, and after anesthetized by intraperitoneal injection of sodium pentobarbital, the brains were removed, coronally cut into two parts, and one part was fixed in 4 % paraformaldehyde; in the other part, brain tissue from the ischemic penumbra was collected, ground in saline, prepared as 10 % homogenate, centrifuged to obtain supernatant, and tested for IL-1β and IL-18 levels by ELISA.
HE staining: Brains fixed in Paraformaldehyde (PFA), embedded in paraffin, and cut at 4 μm-thick sections, and routine HE staining was performed to observe the morphological changes of neurons in the hippocampal CA1 region in the ischemic penumbra.
Immunofluorescence co-staining: Paraffin sections of brain tissues were obtained and blocked with 5 % Bovine Serum Albumin (BSA) for 15 min. Sections were incubated overnight at 4° with the following primary antibodies; goat anti-IBA1 (1:300), rabbit anti-caspase-1 (1:100), rabbit anti-NLRP3 (1:100) and, on alternate days, with fluorescent secondary antibodies; donkey anti-goat IgG (Alexa Fluor® 488, 1:300), goat anti-rabbit IgG (Alexa Fluor® 647, 1:300) and goat anti-rabbit IgG (Alexa Fluor® 647, 1:300) at room temperature (37°) in the dark sections were incubated for 1 h, stained with 4',6-Diamidino- 2-Phenylindole (DAPI) for 10 min, dried, mounted and examined by fluorescence microscopy and images were analyzed using Image Pro Plus 6.0.
Western blot was used to detect NLRP3 pyroptosis related protein expression: Brain ischemic penumbra tissue from the remaining six rats in each group was separated, total protein was extracted from Radioimmunoprecipitation Assay (RIPA) lysate, and after determination of protein concentration, protein samples were separated by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) (30 μg). After membrane permeabilization and blocking for 1 h, the membranes were incubated with primary antibodies (NLRP3, pro caspase-1, cleaved caspase-1 (P10 subunit, P20 subunit), IL-1β and β-actin) overnight at 4°. Alternate days, the membranes were incubated with secondary antibodies for 1 h at room temperature, developed and protein band gray values were analyzed by ImageJ software and calculated the amount of protein expression relative to β-actin.
Statistical analysis:
All data are presented as mean±standard deviation (x̄±s). One way Analysis of Variance (ANOVA) was used to analyze statistical significance, and Tukey's multiple comparison tests was used for differences between groups. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) Statistics 22.0 software. p<0.05 indicated that the difference was statistically significant.
Results and Discussion
Compared with the sham operated group, the neurological deficit score increased in the model group (p<0.05); compared with the model group, the MCC950 group, propofol-L and propofol-H groups showed decreased neurological deficit scores (p<0.05), and the differences between the propofol-L and propofol-H groups were statistically significant (p<0.05) as shown in Table 1.
| Group | Dose (mg/kg) | Neurological deficit scoring |
|---|---|---|
| Sham | 0.00±0.00 | |
| Model | 2.53±0.20a | |
| MCC950 | 10 | 1.58±0.15ab |
| Propofol-H | 10 | 1.65±0.14ab |
| Propofol-L | 20 | 2.07±0.18abcd |
Table 1: Comparison of Neurological Deficit Scores among Rats in Each Group (X͞±S, N=18)
Notes: Compared with Sham group, ap<0.05; compared with model group, bp<0.05; compared with the MCC950 group; cp<0.05 and compared with the propofol-H group, dp<0.05
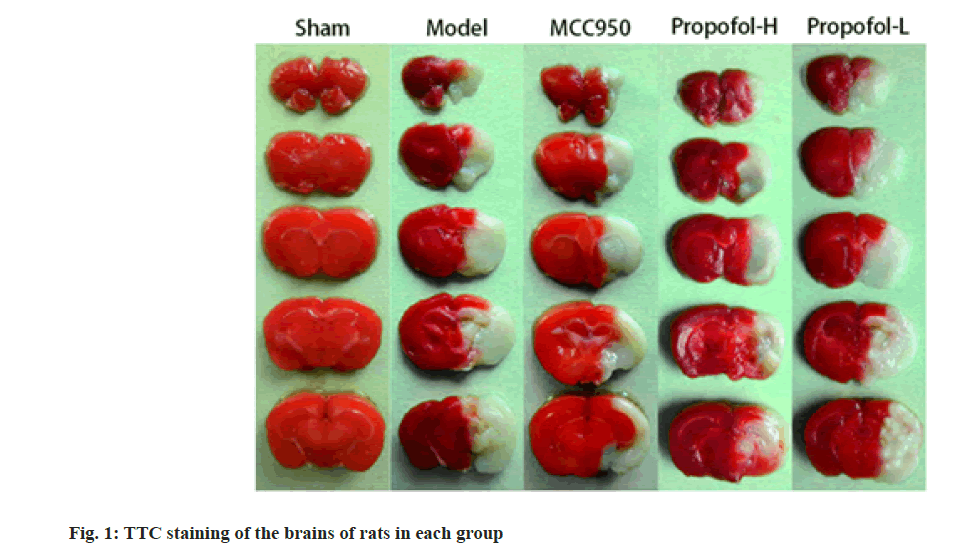
Compared with the sham operated group, the cerebral infarction volume of rats in the model group was increased (p<0.05); compared with the model group, the MCC950 group and the propofol-L and propofol-H groups showed reduced cerebral infarction volumes (p<0.05), and the differences between the propofol-L and propofol-H groups were statistically significant (p<0.05); there was no significant difference in cerebral infarction volume between MCC950 group and propofol-H group (p>0.05) as shown in fig. 1 and Table 2.
| Group | Dose (mg/kg) | Percentage of cerebral infarct volume (%) |
|---|---|---|
| Sham | 0.00±0.00 | |
| Model | 30.51±2.30a | |
| MCC950 | 10 | 15.92±1.64ab |
| Propofol-H | 10 | 16.37±1.52ab |
| Propofol-L | 20 | 26.25±2.17abcd |
Table 2: Comparison of Cerebral Infarct Volumes among Rats in Each Group (X̄±S, N=6)
Notes: Compared with Sham group, ap<0.05; compared with model group, bp<0.05; compared with the MCC950 group; cp<0.05 and compared with the propofol-H group, dp<0.05
Compared with sham operated group, IL-1β and IL-18 levels in model group increased (p<0.05); compared with the model group, the MCC950 group and propofol-L and propofol-H groups had lower levels of IL-1β and IL-18 in the brain tissue (p<0.05), and the differences between propofol-L and propofol-H groups were statistically significant (p<0.05); compared with MCC950 group, there was no significant difference in IL-1β and IL-18 levels in brain tissue of propofol-H group (p>0.05) as shown in Table 3.
| Group | IL-1β | IL-18 |
|---|---|---|
| Sham | 120.14±11.91 | 85.21±9.35 |
| Model | 334.58±25.85a | 190.45±16.28a |
| MCC950 | 155.32±16.93ab | 118.09±10.17ab |
| Propofol-H | 160.11±14.82ab | 125.68±12.12ab |
| Propofol-L | 289.27±20.46abcd | 168.94±15.54abcd |
Table 3: Comparison of Il-1β And Il-18 Levels in Brain Tissue of Rats in Each Group (X̄±S, Pg/Ml, N=6)
Notes: Compared with Sham group, ap<0.05; compared with model group, bp<0.05; compared with the MCC950 group; cp<0.05 and compared with the propofol-H group, dp<0.05
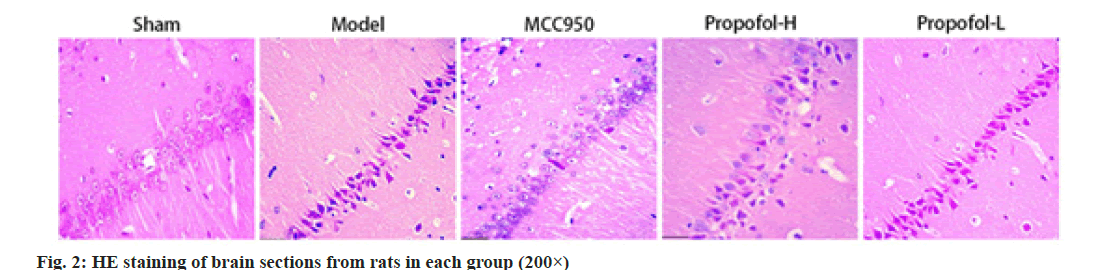
No obvious damage was observed to the cells in the hippocampal CA1 region of the ischemic penumbra in the brain of sham operated group rats; the hippocampus of rats in the model group showed pyknotic deep staining of nuclei, the arrangement of nerve cells was loose, the number was reduced, and vacuolization like changes were present; the MCC950, propofol-L and propofol-H groups ameliorated the pathological lesions to various degrees compared with the model group, with an increased number of neurons and more uniform staining; moreover, the degree of pathological improvement in the hippocampal CA1 region of the propofol-H group was close to that of the MCC950 group as shown in fig. 2.
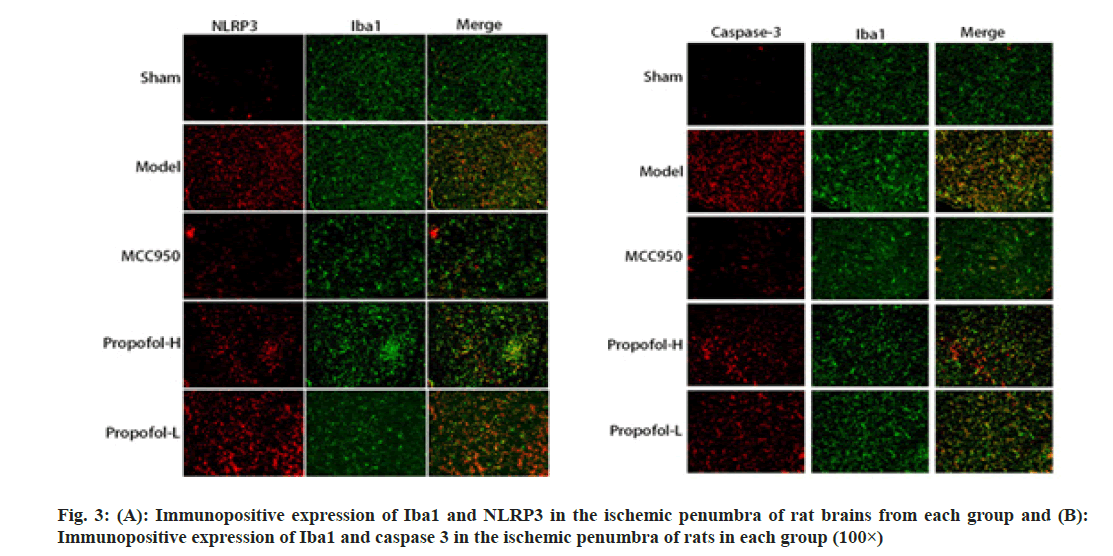
Compared with the sham operated group, the immunopositive co-expression of IBA1/NLRP3 and IBA1/caspase 1 was significantly increased in the ischemic penumbra of model group rats (p<0.05); compared with the model group, immunopositive co-expression of IBA1/NLRP3 and IBA1/caspase 1 was significantly decreased in the MCC950 group, propofol-L and propofol-H groups (p<0.05), and the differences between propofol-L and propofol-H groups were statistically significant (p<0.05); there were no significant differences in the immunopositive expression of NLRP3, caspase-1 and IBA1 in the propofol-H group compared with the MCC950 group (p>0.05) as shown in fig. 3 and Table 4.
| Group | IBA1/NLRP3 | IBA1/caspase 1 |
|---|---|---|
| Sham | 2.94±0.25 | 5.17±0.44 |
| Model | 36.58±3.14a | 49.34±3.68a |
| MCC950 | 21.95±2.06ab | 25.11±2.43ab |
| Propofol-H | 23.04±2.25ab | 27.85±2.12ab |
| Propofol-L | 30.76±2.73abcd | 38.62±3.05abcd |
Table 4: Immunopositive Expression of Nlrp3, Caspase-1 and Iba1 in the Ischemic Penumbra of the Brains of Rats in Each Group (X̄±S, N=6)
Notes: Compared with Sham group, ap<0.05; compared with model group, bp<0.05; compared with the MCC950 group; cp<0.05 and compared with the propofol-H group, dp<0.05
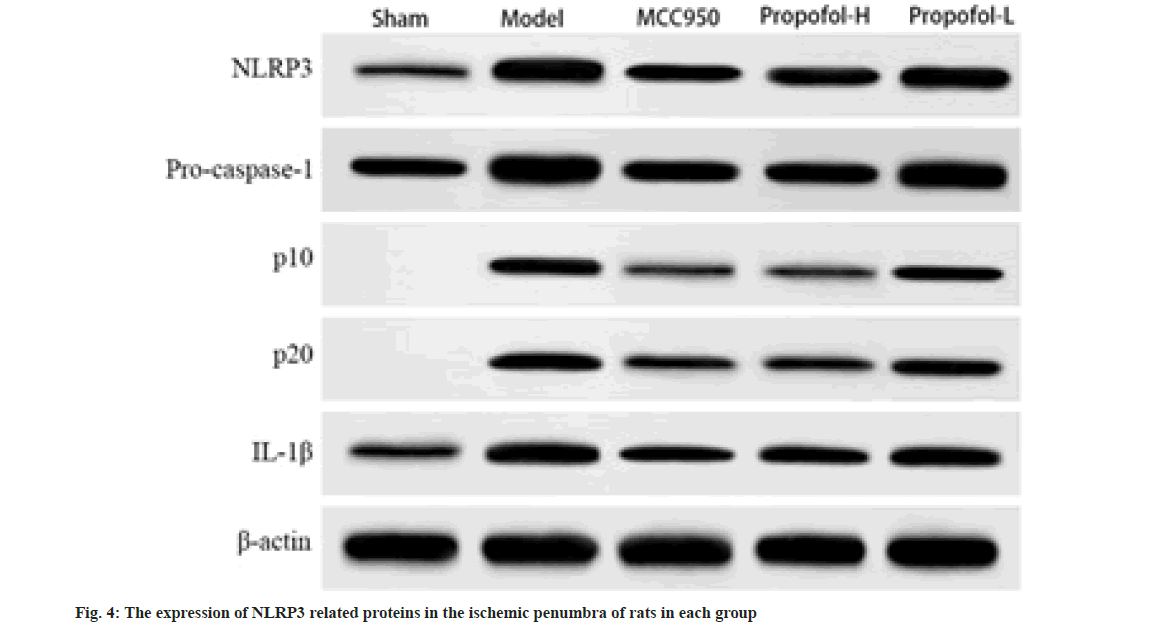
Western blot showed that there was no expression of cleaved caspase-1 p10 or p20 proteins in the sham operated group, and the protein expressions of NLRP3, pro caspase-1, p10, p20 and IL-1β in the ischemic penumbra of rats of the model group were significantly increased compared with the sham operated group); compared with the model group, the MCC950 group, propofol-L and propofol-H groups had significantly lower expression of the above proteins (p<0.05), and the differences between the propofol-L and propofol-H groups were statistically significant (p<0.05); compared with the MCC950 group, there was no significant difference in the expression of the above proteins in the propofol-H group (p>0.05) as shown in fig. 4 and Table 5.
| Group | NLRP3 | Pro-caspase 1 | p10 | p20 | IL-1β |
|---|---|---|---|---|---|
| Sham | 0.12±0.01 | 0.35±0.04 | 0 | 0 | 0.18±0.02 |
| Model | 0.56±0.04a | 0.83±0.07a | 0.33±0.03a | 0.42±0.04a | 0.52±0.04a |
| MCC950 | 0.31±0.03ab | 0.48±0.05ab | 0.15±0.02ab | 0.19±0.02ab | 0.29±0.03ab |
| Propofol-H | 0.33±0.04ab | 0.50±0.06ab | 0.14±0.01ab | 0.21±0.03ab | 0.31±0.04ab |
| Propofol-L | 0.48±0.05abcd | 0.69±0.07abcd | 0.27±0.03abcd | 0.32±0.04abcd | 0.42±0.05abcd |
Table 5: The Expression of Nlrp3 Related Proteins in the Ischemic Penumbra of Rat Brains from Each Group (X̄±S, N=6)
Notes: Compared with Sham group, ap<0.05; compared with model group, bp<0.05; compared with the MCC950 group; cp<0.05 and compared with the propofol-H group, dp<0.05
The MCAO model is generally accepted as the standard animal model of focal cerebral ischemia, and the suture-occluded method is a common method to prepare MCAO reperfusion model with simple operation. The Zea-Longa neurological deficit score is a reliable evaluation criterion for the success of a rat model of focal cerebral ischemia. The results of the present study showed that rats after MCAO exhibited significant neurological deficits, and significant cerebral infarction was seen in the ischemic area; propofol is effective in reducing the cerebral infarct volume after CIR, thereby improving neurological deficits, which is consistent with previous findings. Moreover, in this study, we also found that propofol pretreatment significantly reduced IBAL/NLRP3 and IBA1/caspase 1 immunopositive expression in the ischemic penumbra and inhibited microglial pyroptosis and NLRP3 inflammasome induced inflammation.
Inflammation is thought to play a crucial role in the progression of CIR injury. Pyroptosis is a proinflammatory programmed cell death modality mediated by caspases (caspase-1 and caspase-4/5/11), among them, caspase-1 dependent activation is the classical pyroptosis pathway. Pyroptosis differs from apoptosis in that pyroptosis is mediated by caspase 1 and apoptosis is mediated by caspase 3. Studies have reported that caspase 1 is highly expressed in microglia as observed in the early stage of ischemic stroke[16]; Wang et al.[17] found that melatonin reduced MCAO induced apoptosis of microglia positive for caspase-1 expression, reduced the cerebral infarction size and improved neurological dysfunction, indicating a close relationship between microglial pyroptosis and neurotoxicity in ischemic brain injury. In the present study, IBA1/caspase 1 immunopositive expression was increased in the ischemic penumbra brain after MCAO, which was accompanied by neurological deficits, increased cerebral infarct volume and neuronal loss, illustrating that microglial pyroptosis was increased in the ischemic penumbra of MCAO rats. However, pretreatment with propofol decreased IBA1/caspase 1 immunopositive coexpression. These results suggest that propofol pretreatment may inhibit microglial pyroptosis in the ischemic penumbra to promote neuroprotection.
Proinflammatory cytokines IL-1β and IL-18 are important damage factors in CIR, and its secretion is regulated by activated caspase-1, whereas caspase 1 maturation and release are tightly regulated by the NLRP3 inflammasome[18]. The NLRP3 inflammasome consists of the NLRP3 protein, ASC with the caspase 1 precursor (pro-caspase 1), and the active core is the NLRP3 protein. Studies have demonstrated that NLRP3 inflammasome is activated in cerebral ischemia, and after activation, pro-caspase-1 is cleaved to active cleaved caspase-1 (P10, P20) and cleaves pro-IL-1β and pro-IL-18 to mature IL-1β and IL-18[19]. In the central nervous system, the NLRP3 inflammasome is predominantly expressed in microglia; Zhao et al.[20] showed in MCAO induced mice and OGD/deoxygenation treated BV-2 cells that IBA1/NLRP3 immunopositive co-expression was significantly increased, IL-1β and IL-18 levels increased, which inhibits microglial activation and NLRP3 inflammasome expression, reduces IL- 1β and IL-18 levels, and alleviates the damage of CIR, indicating that NLRP3 mediated canonical pyroptosis pathway may be a key link to promote microglial pyroptosis and aggravate brain injury. Our results showed that propofol pretreatment reduced IBA1/NLRP3 immunopositive co-expression and decreased IL-1β and IL-18 levels in the brain tissue. Western blot results also showed that NLRP3, pro caspase-1, p10, p20 and IL-1β protein expressions in the ischemic penumbra of MCAO rat brains after propofol pretreatment were markedly reduced, and the inhibitory effect on NLRP3 was close to that of the NLRP3 inhibitor MCC950; these results suggest that the neuroprotective effect of propofol is associated with the inhibition of NLRP3 inflammasome activation and microglial pyroptosis in the ischemic penumbra.
Taken together, the neuroprotective effect of propofol pretreatment on CIR injury may be associated with the inhibition of NLRP3 inflammasome mediated microglial pyroptosis in the ischemic penumbra. There are some limitations in this study, first, we only investigated the short-term effects of propofol pretreatment; second, the results of the two propofol dose groups were quite different, which indicated that there might be a certain dose-effect relationship of propofol, and whether there was a dose-dependent manner required the addition of different doses for further research; in addition, whether the noncanonical pyroptosis pathway (caspase-4/5/11) is involved in the neuroprotective effects of propofol preconditioning, still needs to be explored.
Author’s contributions:
Pengdan Ying and Shurong Liu have contributed equally to this work.
References
- Bu J, Shi S, Wang HQ, Niu XS, Zhao ZF, Wu WD, et al. Acacetin protects against cerebral ischemia-reperfusion injury via the NLRP3 signaling pathway. Neural Regen Res 2019;14(4):605-12.
[Crossref] [Google Scholar] [PubMed]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020;141(9):e139-596.
[Crossref] [Google Scholar] [PubMed]
- Akbari G. Role of zinc supplementation on ischemia/reperfusion injury in various organs. Biol Trace Elem Res 2020;196(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Liu A, Zhang W, Wang S, Wang Y, Hong J. HMGB-1/RAGE signaling inhibition by dioscin attenuates hippocampal neuron damage induced by oxygen-glucose deprivation/reperfusion. Exp Ther Med 2020;20(6):231.
[Crossref] [Google Scholar] [PubMed]
- Tang X, Cheng D. Association between microglial pyroptosis and ischemic stroke. Chin J Cerebrovasc Dis 2019;21(11):1230-2.
- She Y, Hu Y, Zhang J. Involvement of pyroptosis in cerebral ischemia-reperfusion injury: A preliminary study. Chin J Pathophysiol 2019;35(8):1379-86.
- Hu J, Zeng C, Wei J, Duan F, Liu S, Zhao Y, et al. The combination of Panax ginseng and Angelica sinensis alleviates ischemia brain injury by suppressing NLRP3 inflammasome activation and microglial pyroptosis. Phytomedicine 2020;76:153251.
[Crossref] [Google Scholar] [PubMed]
- Shen L, Wu Z, Liu X. Effect of carbenoxolone injection on microglial activation and differentiation of different subtypes after cerebral ischemia/reperfusion in rats. J Dali Univ 2020;5(2):56-60.
- Wang Y, Tian D, Wei C, Cui V, Wang H, Zhu Y, et al. Propofol attenuates α-synuclein aggregation and neuronal damage in a mouse model of ischemic stroke. Neurosci Bull 2020;36(3):289-98.
- Wu MB, Ma B, Zhang TX, Zhao K, Cui SM, He SC. Propofol improves intestinal ischemia-reperfusion injury in rats through NF-κB pathway. Eur Rev Med Pharmacol Sci 2020;24(11):6463-9.
[Crossref] [Google Scholar] [PubMed]
- Kang K, Huang C, Chen L. A clinical study of propofol combined with dexmedetomidine in patients with cerebral ischemia-reperfusion injury. Chin J Clin Pharmacol 2020;36(10):1205-7.
- Liu Z, Meng Y, Miao Y, Yu L, Yu Q. Propofol reduces renal ischemia/reperfusion-induced acute lung injury by stimulating sirtuin 1 and inhibiting pyroptosis. Aging 2021;13(1):865-76.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Li K, Chen P, Pan J, Li X, Zhao G. Propofol attenuates inflammatory damage via inhibiting NLRP1-Casp1-Casp6 signaling in ischemic brain injury. Biol Pharm Bull 2020;43(10):1481-9.
[Crossref] [Google Scholar] [PubMed]
- Wen Y, Ju K. Propofol exerts neuroprotective effects on rats with cerebral ischemia through the NLRP3/caspase-1 pathway. Chin Pharm J 2020;23(6):1081-6.
- Huang Y, Wang J, Du L. Puerarin regulated AMPK-mTOR signaling pathway to inhibit autophagy to improve cerebral ischemia reperfusion injury in rats study. Chin Tradit Herbal Drugs 2019;50(13):3127-33.
- Tang X, Cheng D. Association between microglial pyroptosis and ischemic stroke. Chin J Cerebrovasc Dis 2019;21(11):1230-2.
- Wang K, Ru J, Zhang H, Chen J, Lin X, Lin Z, et al. Melatonin enhances the therapeutic effect of plasma exosomes against cerebral ischemia-induced pyroptosis through the TLR4/NF-κB pathway. Front Neurosci 2020;14:848.
[Crossref] [Google Scholar] [PubMed]
- Pereira CA, Carlos D, Ferreira NS, Silva JF, Zanotto CZ. Mitochondrial DNA promotes NLRP3 inflammasome activation and contributes to endothelial dysfunction and inflammation in type 1 diabetes. Front Physiol 2020;10:1557.
[Crossref] [Google Scholar] [PubMed]
- Li L, Ismael S, Nasoohi S, Sakata K, Liao FF, McDonald MP, et al. Thioredoxin-interacting protein (TXNIP) associated NLRP3 inflammasome activation in human Alzheimer’s disease brain. J Alzheimer's Dis 2019;68(1):255-65.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Piao X, Wu Y, Liang S, Han F, Liang Q et al. Cepharanthine attenuates cerebral ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced inflammation and oxidative stress via inhibiting 12/15-LOX signaling. Biomed Pharmacother 2020;127:110151.
[Crossref] [Google Scholar] [PubMed]