- *Corresponding Author:
- Jiali Wen
Department of Anesthesiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310000, China
E-mail: agawen0723@163.com
| Date of Received | 18 December 2021 |
| Date of Revision | 05 October 2022 |
| Date of Acceptance | 16 August 2023 |
| Indian J Pharm Sci 2023;85(4):1110-1117 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of propofol on oxidative stress injury and silent information regulator 1/forkhead box protein 1 pathway of Kupffer cells induced by hydrogen peroxide. Kupffer cells were cultured in vitro, cell injury model was established by hydrogen peroxide induction, and it was divided into three groups; hydrogen peroxide injury group (300 μmol·l-1), propofol low (12.5 μg.ml-1), propofol medium (25.0 μg.ml-1), and propofol high (50.0 μg·ml-1) groups, in addition, Kupffer cells without any treatment were normal control group. Compared with normal control group ((100.00±0.00) %, (98.19±2.48), (3.76±0.21) pg/ml, (2.46±0.25) ng/l, (2.47±0.16) pmol/mg, (1.01±0.09), (8.31±0.23), (1.03±0.08), (1.12±0.11), (2.18±0.13)), the Kupffer cell survival rate (56.24±2.12) %, tetramethylrhodamine ethyl ester fluorescence intensity (54.57±2.64), contents of superoxide dismutase (2.51±0.19) pg/ml, catalase (1.23±0.14) ng/l, glutathione peroxidase in cell supernatant (1.31±0.11) pmol/mg, silent information regulator 1 (0.21±0.02), forkhead box protein 1 messenger ribonucleic acid (0.99±0.03) and protein expressions [(0.19±0.02), (0.11±0.01)) decreased significantly in hydrogen peroxide group (p<0.05), and the content of malondialdehyde (4.56±0.37) mol/l in supernatant increased significantly (p<0.05); compared with hydrogen peroxide group, the Kupffer cell survival rate ((64.79±2.49) %, (72.62±2.53) %, (84.91±2.87) %), tetramethylrhodamine ethyl ester fluorescence intensity [(67.28±2.51), (80.65±2.64), (94.19±3.05)), contents of superoxide dismutase ((2.89±0.23) pg/ml, (3.24±0.15) pg/ml, (3.57±0.18) pg/ml), catalase ((1.57±0.18) ng/l, (1.88±0.19) ng/l, (2.23±0.17) ng/l), glutathione peroxidase in cell supernatant ((1.76±0.15) pmol/mg, (2.03±0.16) pmol/mg, (2.39±0.17) pmol/mg), silent information regulator 1 ((0.39±0.03), (0.52±0.04), (0.86±0.05)), forkhead box protein 1 messenger ribonucleic acid ((2.85±0.27), (4.11±0.22), (6.19±0.36)) and protein expressions (silent information regulator 1: (0.48±0.04), (0.71±0.06), (1.01±0.07)), (forkhead box protein 1: (0.45±0.06), (0.64±0.07), (0.87±0.09)) increased significantly in turn of propofol low group, propofol medium group and propofol high group (p<0.05), and the content of malondialdehyde ((4.18±0.35) mol/l, (3.62±0.14) mol/l, (2.87±0.17) mol/l) in supernatant decreased significantly in turn (p<0.05). Propofol can alleviate the oxidative stress injury of Kupffer cells induced by hydrogen peroxide and promote their survival, which may be related to the regulation of silent information regulator 1/forkhead box protein 1 pathway.
Keywords
Propofol, silent information regulator 2 related enzyme 1/forkhead box O1 pathway, Kupffer cell, oxidative stress injury
Hepatic Ischemia/Reperfusion (I/R) injury is a key factor affecting the success of surgery such as lobectomy and liver transplantation, which can increase postoperative mortality[1,2]. Kupffer cells are a special kind of macrophages that are located in the liver sinusoids and account for 80 %-90 % of total body mononuclear macrophages, which are clean guards for the liver, and over activation of Kupffer cells can produce a large number of oxygen free radicals and inflammation related factors, aggravating tissue damage[3-5]. Therefore, avoiding the over activation of Kupffer cells is of great importance to clinically reduce hepatic I/R injury. Studies have reported that certain anesthetics and sedative drugs such as Propofol (Pro) and dexmedetomidine have better protective effects against visceral I/R injury[6,7]. Pro, one of the commonly used intravenous general anesthesia drugs in clinic, can be used to sedate anesthesia, and recent studies have found that Pro also has antiemetic, anxiolytic and antioxidant stress effects[8-10]. But the effect of Pro on oxidative stress in Kupffer cells has not been reported. The Silent Information Regulator 2-Related Enzyme 1/Forkhead Box O1 (Sirt1/FoxO1) pathway has been implicated in cell proliferation, apoptosis, mitochondrial energy metabolism and anti-oxidative stress effects[11-13]. Therefore, in this study, we used Hydrogen peroxide (H2O2) induced rat Kupffer cells to establish a cell model of oxidative stress, and to explore the effect of Pro on Kupffer cell injury repair and Sirt1/FoxO1 pathway under oxidative stress condition, in order to reveal its protective mechanism against oxidative stress injury in Kupffer cells.
Materials and Methods
Cells, main reagents and instruments:
Rat liver Kupffer cells (Cat No: HTX1973, American Type Culture Collection) were purchased from Otwo Biotech (Shenzhen) Inc.; Roswell Park Memorial Institute (RPMI) 1640 medium (Cat No: 21870084), fetal bovine serum (Cat No: 10099141) were purchased from Gibco, United States of America (USA); 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) Kit (Cat No: V13154), microRNA (miRNA) extraction kit (Cat No: AM1561) were purchased from Invitrogen, USA; PrimeScript™ RT reagent kit (Cat No: RR037A), TB Green® Premix Ex Taq™ II (Cat No: RR820A) was purchased from TaKaRa Bio, Inc.; the primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd.; Malonaldehyde (MDA) assay kit (Cat No: P8242), Catalase (CAT) assay kit (Cat No: BC0205) were purchased from Beijing Solarbio Science and Technology Co., Ltd; Superoxide Dismutase (SOD) assay kit (Lot No: 20170401), Glutathione Peroxidase (GSH-Px) assay kit (Lot No: 20160909) were purchased from Sangon Biotech (Shanghai) Co., Ltd.; Tetramethylrhodamine Ethyl Ester (TMRE) mitochondrial membrane potential assay kit, rabbit primary antibodies anti- Sirt1, anti-FoxO1, anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), and secondary antibodies goat anti-rabbit Immunoglobulin G (IgG) (Cat No: ab113852, ab220807, ab39670, ab181602 and ab205718) were purchased from Abcam, United Kingdom (UK); Olympus light microscope (Model: IX75) was purchased from Olympus, Japan and microplate reader (MODEL550) was purchased from Bio-Rad, USA.
Method:
Cell culture and grouping: Cells were routinely suspended, seeded in RPMI 1640 medium, incubated in an incubator at an appropriate growth density, digested with 0.25 % trypsin, and passaged. Kupffer cells that grew well were divided into five groups; Normal Control (NC group), Kupffer cells were cultured normally without any intervention; H2O2injury group (H2O2 group), Kupffer cells were treated with 300 μmol·l-1 H2O2 for 24 h[14]; Pro Low dose group (Pro-L group), Pro Middle dose group (Pro-M group), Pro High dose group (Pro-H group), Kupffer cells were treated by adding Pro 12.5 μg·mll-1, 25.0 μg·ml-1, 50.0μ g·ml-1 respectively after pretreatment for 24 h[15], 300 μmol·l-1 H2O2 was added to continue the treatment for 24 h.
Cell viability assay: Kupffer cell viability was assessed by the MTT assay, which was performed by adding 20 μl of MTT solution 4 h before the end of Kupffer cell culture in each group, with specific reference to the kit instructions. The cell absorbance (Optical Density (OD)) value of each well at a wavelength of 570 nm was measured with a microplate reader, and the cell viability (%)=(OD value of each treatment group/OD value of NC group)×100 %.
Mitochondrial membrane potential observation: The mitochondrial membrane potential was detected by TMRE based mitochondrial membrane potential assay kit, and the specific procedures were performed according to the kit instructions. The TMRE fluorescence intensities at excitation/emission wavelengths of 549/575 nm were read separately, and the ratio to the basal value was calculated.
SOD, MDA, CAT, GSH-Px of cell supernatant: The supernatant of Kupffer cells from each group was collected and MDA, SOD, CAT and GSH-Px levels were determined biochemically, the specific procedures were performed according to the kit instructions.
Cellular Sirt1 and messenger Ribonucleic Acid (mRNA) expression: Total RNA was extracted from cells, reverse transcribed to complementary Deoxyribonucleic Acid (cDNA), and stored at -20° until use. Sirt1 and FoxO1 mRNA were amplified by Real-Time quantitative Polymerase Chain Reaction (RT-qPCR) using the following conditions; 95° for 30 s; 95° for 5 s, 60° for 34 s, 40 cycles, the specific operation was performed according to the kit instructions. Sirt1 upstream primer sequences 5′-TTGGCACCGATCCTCGAAC-3′, reverse primer sequences 5′-CCCAGCTCCAGTCAGAACTAT-3′; FoxO1 upstream primer sequences 5′-CCTACCTTGGCACGAGAGTG-3′, reverse primer sequences CAGCAGAAGCAGATGGGATT; the internal reference GAPDH upstream primer sequences 5′-ACCACCATGGAGAAGGCTGG-3′, reverse primer sequences 5′-CTCAGTGTAGCCCAGGATGC-3′. The 2-ΔΔCT method was used to analyze the relative expression amount of Sirt1 and FoxO1 mRNA.
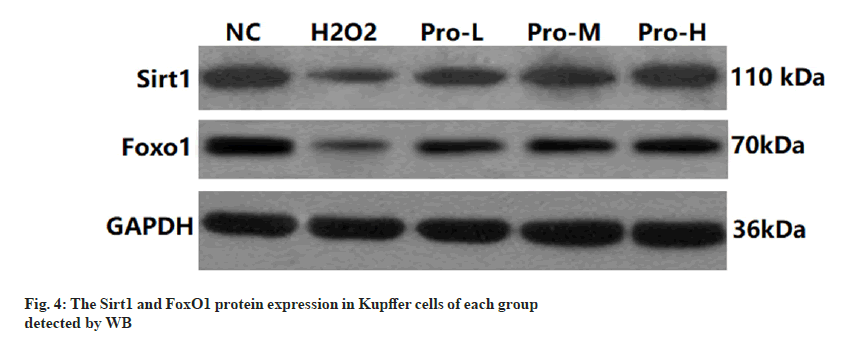
Protein expression of Sirt1 and FoxO1 in cells: The protein expressions of Sirt1 and FoxO1 were determined by Western Blot (WB). RIPA strong lysis buffer was used to lyse and extract total cellular protein, and the protein concentration was determined by Bicinchoninic Acid (BCA) assay and stored at -80°. Protein samples 50 μg were taken for electrophoresis in 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), proteins were semi-dry transferred to Polyvinylidene Difluoride (PVDF) membranes, blocked for 2 h at room temperature, anti-Sirt1 (1:1000), anti-FoxO1 (1:1000), anti-GAPDH (1:5000) antibodies were added, and the membranes were incubated overnight at 4°, washed with Tris-Buffered Saline with 0.1 % Tween® 20 (TBST) detergent, and secondary antibody IgG (dilution ratio 1:10000) was added and incubated for 1 h at room temperature, developed, exposed, observed and photographed and analyzed for band gray levels, so ImageJ software was used for gray value quantification, and the relative expression of target protein was measured as the ratio of band gray value between target protein and internal reference protein GAPDH.
Statistical processing:
Data were analyzed with Statistical Package for the Social Sciences (SPSS) 25.0. Data that conformed to normal distribution were expressed as x? ±s, and univariate Analysis of Variance (ANOVA) was used for comparison of data among multiple groups, and Student–Newman–Keuls (SNK-q) test was used for further pairwise comparison. p<0.05 was considered significant.
Results and Discussion
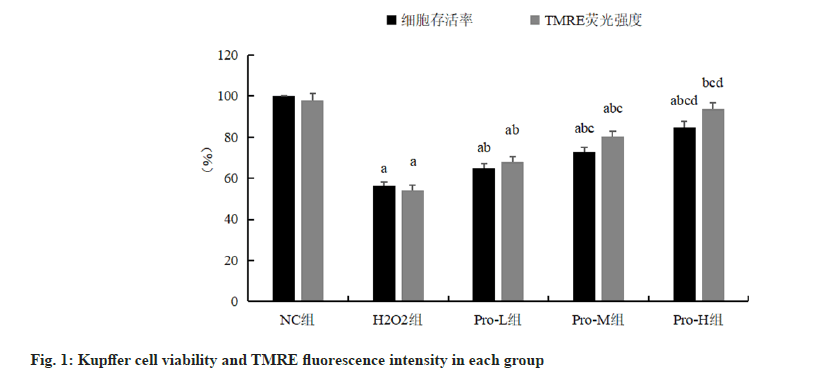
AKupffer cell viability in the H2O2 group was significantly lower than that in the NC group (p<0.05); compared with H2O2 group, Kupffer cell viability in Pro-L group, Pro-M group, Pro-H group increased in turn (p<0.05), as shown in fig. 1 and Table 1.
| Group | Concentration (µmol·l-1) | Cell viability (%) | TMRE fluorescence intensity |
|---|---|---|---|
| NC | 100.00±0.00 | 98.19±2.48 | |
| H2O2 | 300 | 56.24±2.12a | 54.57±2.64a |
| Pro-L | 12.5 | 64.79±2.49ab | 67.28±2.51ab |
| Pro-M | 25.0 | 72.62±2.53abc | 80.65±2.64abc |
| Pro-H | 50.0 | 84.91±2.87abcd | 94.19±3.05bcd |
| F | 350.102 | 280.712 | |
| p | <0.01 | <0.01 |
Note: ap<0.05, compared with the NC group; bp<0.05, compared with the H2O2 group; cp<0.05, compared with the Pro-L group and dp<0.05 compared with the Pro-M group.
Table 1: Comparison Of Kupffer Cell Viability And Tmre Fluorescence Intensity In Each Group (N=6,  ).
).
Compared with the NC group, the TMRE fluorescence intensity of Kupffer cells in the H2O2 group was significantly reduced (p<0.05); compared with H2O2 group, the TMRE fluorescence intensity of Kupffer cells in Pro-L group, Pro-M group, Pro-H group increased in turn (p<0.05), as shown in fig. 1 and fig. 2.
Compared with the NC group, the contents of MDA in the supernatant of Kupffer cells in the H2O2 group were significantly increased (p<0.05), and the contents of SOD, CAT and GSH-Px were significantly decreased (p<0.05); compared with H2O2 group, the contents of MDA in the supernatant of Kupffer cells in Pro-L group, Pro-M group, and Pro-H group decreased sequentially (p<0.05) and SOD, CAT and GSH-Px contents increased sequentially (p<0.05) as shown in Table 2.
| Group | Concentration (mmol·l-1) | MDA (mol/l) | SOD (pg/ml) | CAT (ng/l) | GSH-Px (pmol/mg) |
|---|---|---|---|---|---|
| NC | 2.18±0.13 | 3.76±0.21 | 2.46±0.25 | 2.47±0.16 | |
| H2O2 | 300 | 4.56±0.37a | 2.51±0.19a | 1.23±0.14a | 1.31±0.11a |
| Pro-L | 12.5 | 4.18±0.35ab | 2.89±0.23ab | 1.57±0.18ab | 1.76±0.15ab |
| Pro-M | 25.0 | 3.62±0.14abc | 3.24±0.15abc | 1.88±0.19abc | 2.03±0.16abc |
| Pro-H | 50.0 | 2.87±0.17abcd | 3.57±0.18bcd | 2.23±0.17bcd | 2.39±0.17abcd |
| F | 86.317 | 40.856 | 40.835 | 59.325 | |
| p | <0.01 | <0.01 | <0.01 | <0.01 |
Note: ap<0.05, compared with the NC group; bp<0.05, compared with the H2O2 group; cp<0.05, compared with the Pro-L group and dp<0.05 compared with the Pro-M group
Table 2: Comparison Of The Contents Of Sod, Mda, Cat And Gsh-Px In The Supernatant Of Kupffer Cells In Each Group (N=6, 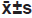 )
)
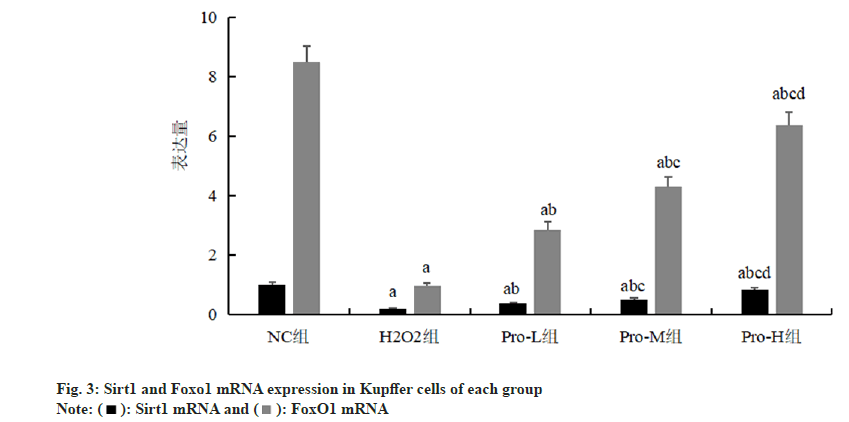
Compared with the NC group, the expression of Sirt1 and FoxO1 mRNA in Kupffer cells was significantly decreased in the H2O2 group (p<0.05); compared with H2O2 group, the Sirt1 and FoxO1 mRNA expression in Kupffer cells of Pro-L group, Pro-M group and Pro-H group increased in turn (p<0.05) as shown in fig. 3 and Table 3.
| Group | Concentration (mmol·l-1) | Sirt1 mRNA | FoxO1 mRNA |
|---|---|---|---|
| NC | 1.01±0.09 | 8.31±0.23 | |
| H2O2 | 300 | 0.21±0.02a | 0.99±0.03a |
| Pro-L | 12.5 | 0.39±0.03ab | 2.85±0.27ab |
| Pro-M | 25.0 | 0.52±0.04abc | 4.11±0.22abc |
| Pro-H | 50.0 | 0.86±0.05abcd | 6.19±0.36abcd |
| F | 243.489 | 801.602 | |
| p | <0.01 | <0.01 |
Note: ap<0.05, compared with the NC group; bp<0.05, compared with the H2O2 group; cp<0.05, compared with the Pro-L group and dp<0.05 compared with the Pro-M group
Table 3: Comparison Of Sirt1 And Foxo1 Mrna Expression In Kupffer Cells Of Each Group (N=6, 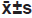 )
)
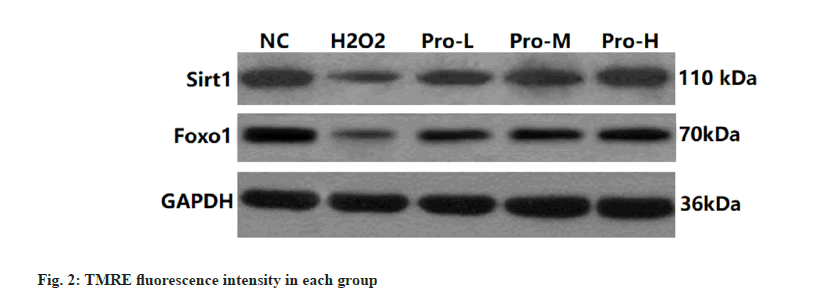
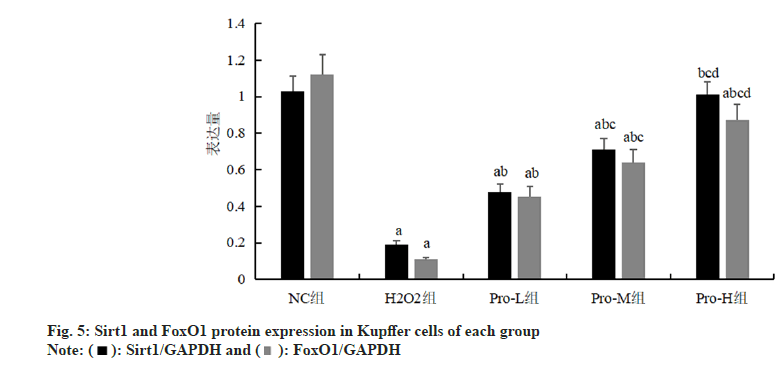
Compared with the NC group, the expression of Sirt1 and FoxO1 protein in Kupffer cells was significantly decreased in the H2O2 group (p<0.05); compared with H2O2 group, the Sirt1 and FoxO1 protein expression in Kupffer cells of Pro-L group, Pro-M group and Pro-H group increased in turn (p<0.05) as shown in fig. 4, fig. 5 and Table 4.
| Group | Concentration (mmol·l-1) | Sirt1 | FoxO1 |
|---|---|---|---|
| NC | 1.03±0.08 | 1.12±0.11 | |
| H2O2 | 300 | 0.19±0.02a | 0.11±0.01a |
| Pro-L | 12.5 | 0.48±0.04ab | 0.45±0.06ab |
| Pro-M | 25.0 | 0.71±0.06abc | 0.64±0.07abc |
| Pro-H | 50.0 | 1.01±0.07bcd | 0.87±0.09abcd |
| F | 227.361 | 156.323 | |
| p | <0.01 | <0.01 |
Note: ap<0.05, compared with the NC group; bp<0.05, compared with the H2O2 group; cp<0.05, compared with the Pro-L group and dp<0.05 compared with the Pro-M group
Table 4: Comparison Of Sirt1 And Foxo1 Protein Expression In Kupffer Cells Of Each Group (N=6,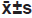 ).
).
Oxidative stress is a key factor in the body's cells undergoing injury and excessive reactive oxygen radicals and lipid peroxides can lead to mitochondrial dysfunction in hepatocytes and induce a large number of proinflammatory factors production. Kupffer cells are important immunocompetent cells in the body, can secrete a variety of cytokines, have phagocytic function, and can also regulate liver parenchymal cell function, but it’s over activation can produce a large number of free radicals and intracellular calcium, aggravating liver tissue damage. MDA is a metabolite of body lipid peroxidation, and its level may reflect the lipid peroxidation progress; SOD, GSH-Px are the peroxidase decomposition enzymes in the body, which can reduce lipid peroxidation and scavenge excessive oxygen free radicals in the body to maintain the normal structure and function of the cell membrane, and their levels are related to the strong and weakness of body's antioxidant capacity; CAT is the hallmark enzyme of peroxisomes and catalyzes H2O2 decomposition. In this study, we found that Kupffer cell viability, TMRE fluorescence intensity, SOD, CAT, and GSH-Px contents were significantly decreased and MDA content was significantly increased in the H2O2 group compared with the NC group, suggesting that Kupffer cells were over activated after H2O2 induction, the levels of antioxidant stress in the cells were decreased, oxidative stress levels were increased, and mitochondrial oxidative stress damage occurred, indicating that this model was induced successfully and can be used for subsequent tests.
Pro is a clinically commonly used short acting intravenous anesthetic that can be used for the induction and maintenance of general anesthesia, and also can alleviate the oxygen radical initiated peroxidation cascade damage reaction by providing a hydrogen atom to react directly with oxygen radicals[16,17]. Liu et al.[18] reported that Pro alleviated H2O2 induced oxidative stress injury in cardiomyocytes by inhibiting mitochondrial and endoplasmic reticulum mediated apoptotic signaling pathway. In this study, we found that, compared with H2O2 group, with the increasing of Pro dosage, Kupffer cell viability, TMRE fluorescence intensity, SOD, CAT and GSH-Px contents increased in turn, MDA content decreased in turn, suggesting that Pro can improve the level of antioxidant stress in cells, alleviate H2O2 induced mitochondrial oxidative stress damage in Kupffer cells, promote their survival and show a dose-dependent manner.
Sirt1, a NAD+ dependent class III histone deacetylase, is highly conserved during evolution, and Sirt1 can decrease FoxO1 acetylation levels through deacetylation and activate FoxO1 transcriptional activity, which plays a role in cellular energy metabolism maintenance, anti-inflammation, anti-oxidative stress and immune regulation[19,20]. Sun et al.[21] showed that Sirt1 acts as an anti-inflammatory factor and participates in the process of Wuweizi asthma decoction to ameliorate symptoms in asthmatic mice. He et al.[22] reported that FoxO1 can regulate autophagy, oxidative stress and mitochondrial dysfunction, and has potential therapeutic value in the treatment of human cholangiocarcinoma. In this study, we found that compared with the NC group, the Sirt1 and FoxO1 mRNA levels and protein expression in Kupffer cells were significantly decreased in the H2O2 group, suggesting that mitochondrial oxidative stress injury in Kupffer cells involves regulation of the Sirt1/FoxO1 signaling pathway. Zhu et al.[23] showed that Pro attenuated oxidative stress injury in cardiomyocytes induced by cobalt chloride, which may act by activating the Sirt1/FoxO1 pathway. In this study, compared with the H2O2 group, the expression of Sirt1 and FoxO1 mRNA and protein in Kupffer cells increased sequentially with the increasing dosage of Pro, which suggested that Pro could upregulate Sirt1 and FoxO1 expression in a dose-dependent manner, relieve the inhibition of Sirt1/FoxO1 signaling pathway in Kupffer cells by H2O2, and enhance the anti-oxidative stress response, and then alleviate oxidative stress injury in Kupffer cells induced by H2O2.
In conclusion, Pro can alleviate the oxidative stress injury and promote the survival of Kupffer cells induced by H2O2, which may be associated with regulating the Sirt1/FoxO1 pathway. This study initially investigated the protective mechanism of Pro against oxidative stress injury in Kupffer cells, and the subsequent animal experiments will be conducted in the hope of providing more valuable references for clinical application.
Acknowledgement:
This research was supported by National Natural Science Foundation of Chongqing (cstc2019jcymsxmx0439) and Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ1726396).
Conflict of interests:
The authors declared no conflict of interests.
References
- Li L, Zheng X, Zheng T, Huang F, Chen J, Tu W. Effects of limb ischemic preconditioning on intestinal injury and brain injury induced by hepatic ischemia-reperfusion in rats. Chin J Anesthesiol 2018;38(1):105-9.
- Gdara NB, Belgacem A, Khemiri I, Mannai S, Bitri L. Protective effects of phycocyanin on ischemia/reperfusion liver injuries. Biomed Pharmacother 2018;102(1):196-202.
- Czech TY, Seki E. Kupffer cell TLR2/3 signaling: A pathway for EGCG amelioration of ethanol-induced hepatic injury. Cell Mol Gastroenterology Hepatol 2020;9(1):187-8.
[Crossref] [Google Scholar] [PubMed]
- Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain Kupffer cell identity. Immunity 2019;51(4):655-70.
[Crossref] [Google Scholar] [PubMed]
- Kermanizadeh A, Brown DM, Stone V. The variances in cytokine production profiles from non-or activated THP-1, Kupffer cell and human blood derived primary macrophages following exposure to either alcohol or a panel of engineered nanomaterials. PloS One 2019;14(8):e0220974.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Chen F, Wu H. Effects of propofol on the expression of toll-like receptor 4 and high mobility group box 1 protein in a rat model of myocardial ischemia-reperfusion injury. J Clin Med Pract 2021;25(3):13-8.
- Shao Y, Mo P, Shen Z. Effects of intravenous pump dexmedetomidine administered before induction of anesthesia and during maintenance of general anesthesia on postoperative hepatic ischemia-reperfusion injury and oxidative stress in colorectal cancer patients. J Chin Phys 2019;21(10):1563-7.
- Zhang Y, Zuo Y, Li B, Xie J, Ma Z, Thirupathi A, et al. Propofol prevents oxidative stress and apoptosis by regulating iron homeostasis and targeting JAK/STAT3 signaling in SH-SY5Y cells. Brain Res Bull 2019;153:191-201.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Yu D. Comparison of the effects of sevoflurane inhalation by concentration decreasing method and propofol remifentanil step-by-step target infusion for painless colonoscopy in elderly patients. J Clin Med Pract 2020;24(9):40-3.
- Ming N, Na HS, He JL, Meng QT, Xia ZY. Propofol alleviates oxidative stress via upregulating lncRNA-TUG1/Brg1 pathway in hypoxia/reoxygenation hepatic cells. J Biochem 2019;166(5):415-21.
[Crossref] [Google Scholar] [PubMed]
- Jing Z, Wang C, Wen S, Jin Y, Meng Q, Liu Q, et al. Phosphocreatine promotes osteoblastic activities in H2O2-induced MC3T3-E1 cells by regulating SIRT1/FOXO1/PGC-1α signaling pathway. Curr Pharm Biotechnol 2021;22(5):609-21.
[Crossref] [Google Scholar] [PubMed]
- Alegre P, Mathias L, Lourenço MA, Santos PP, Gonçalves A, Fernandes AA, et al. Euterpe oleracea mart. (acai) reduces oxidative stress and improves energetic metabolism in myocardial ischemia-reperfusion injury in rats. Arq Bras Cardiol 2019;114:78-86.
[Google Scholar] [PubMed]
- Li D, Liang H, Li Y, Zhang J, Qiao L, Luo H. Allicin alleviates lead-induced bone loss by preventing oxidative stress and osteoclastogenesis via SIRT1/FOXO1 pathway in mice. Biol Trace Element Res 2021;199:237-43.
[Crossref] [Google Scholar] [PubMed]
- Han Z, Li S, Li Y. Antioxidant effect of senescence marker protein 30 in human lens epithelial cell strain SRA01/04 at the initial stage of acute oxidative stress. Recent Adv Ophthalmol 2019;39(2):109-12.
- Sumi C, Okamoto A, Tanaka H, Nishi K, Kusunoki M, Shoji T, et al. Propofol induces a metabolic switch to glycolysis and cell death in a mitochondrial electron transport chain-dependent manner. PloS One 2018;13(2):e0192796.
[Crossref] [Google Scholar] [PubMed]
- Yu W, Gao D, Jin W, Liu S, Qi S. Propofol prevents oxidative stress by decreasing the ischemic accumulation of succinate in focal cerebral ischemia–reperfusion injury. Neurochem Res 2018;43(2):420-9.
[Crossref] [Google Scholar] [PubMed]
- Romans CW, Day TK, Smith JJ. Oxidative red blood cell damage associated with propofol and intravenous lipid emulsion therapy in a dog treated for 5-fluorouracil toxicosis. J Vet Emerg Crit Care 2020;30(4):481-6.
[Crossref] [Google Scholar] [PubMed]
- Liu XR, Cao L, Li T, Chen LL, Yu YY, Huang WJ, et al. Propofol attenuates H2O2-induced oxidative stress and apoptosis via the mitochondria and ER-medicated pathways in neonatal rat cardiomyocytes. Apoptosis 2017;22(5):639-46.
[Crossref] [Google Scholar] [PubMed]
- Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med 2020;24(21):12355-67.
[Crossref] [Google Scholar] [PubMed]
- He J, Zhang A, Song Z, Guo S, Chen Y, Liu Z, et al. The resistant effect of SIRT1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. Biosci Rep 2019;39(5):BSR20190112.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Ren Y, Kong Q. Effects of Wuweizi asthma decoction on SIRT1/Akt signaling and lung function in asthmatic mice. Chin J Pathophysiol 2021;37(3):481-6.
- He W, Zhang A, Qi L, Na C, Jiang R, Fan Z, et al. FOXO1, a potential therapeutic target, regulates autophagic flux, oxidative stress, mitochondrial dysfunction and apoptosis in human cholangiocarcinoma QBC939 cells. Cell Physiol Biochem 2018;45(4):1506-14.
[Crossref] [Google Scholar] [PubMed]
- Zhu S, Wang Z, Chen C. Propofol modulates the Sirt1/FoxO1 pathway to protect against cobalt chloride induced myocardial injury. Chin J Pathophysiol 2020;29(5):1-5.