- *Corresponding Author:
- Jinhua Ding
Department of Breast and Thyroid Surgery, The Affiliated Lihuili Hospital, Ningbo University, Ningbo, Zhejiang 315040
E-mail: cookie800128@sina.com
| Date of Received | 22 November 2021 |
| Date of Revision | 04 March 2022 |
| Date of Acceptance | 05 May 2023 |
| Indian J Pharm Sci 2023;85(3):613-620 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of formononetin on phosphatase and tensin homology deleted on chromosome ten/protein kinase B signaling pathway and angiogenesis of transplanted tumor in nude mice with breast cancer cells. Nude mice with breast cancer cell transplanted tumors were established and randomly divided into control group, low (0.1 mg/g) and medium (0.2 mg/g) doses of formononetin, high (0.4 mg/g) and taxol (10 μg/g) groups, after grouping treatment, the transplanted tumor volumes of nude mice in each group were measured; terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining was used to detect the apoptosis of transplanted tumor cells; the angiogenesis (proportion of cluster of differentiation 31 positive cells) of transplanted tumors was detected by immunohistochemical staining; serum vascular endothelial growth factor levels in nude mice were detected by enzyme-linked immunosorbent assay; the expressions of vascular endothelial growth factor and phosphatase and tensin homology deleted on chromosome ten/protein kinase B pathway related proteins in transplanted tumors were detected by Western blot assay. Compared to the control group, the volumes of transplanted tumors, the proportion of cluster of differentiation 31 positive cells, serum vascular endothelial growth factor levels, vascular endothelial growth factor protein expression, and phosphorylated-protein kinase B/protein kinase B of nude mice were decreased, while the proportion of apoptotic cells and phosphatase and tensin homology deleted on chromosome ten protein expression were increased in a dose-dependent manner (p<0.05) in low, medium and high doses of formononetin or taxol groups; however, there were no differences in all indexes between taxol group and high dose group of formononetin (p>0.05). Formononetin up-regulates phosphatase and tensin homology deleted on chromosome ten expression, decreases protein kinase B phosphorylation and inhibits transplanted tumor growth and angiogenesis of transplanted tumor in nude mice with breast cancer cells.
Keywords
Formononetin, breast cancer cell, transplanted tumor, nude mice, phosphatase and tensin homology deleted on chromosome ten/protein kinase B signaling pathway, angiogenesis
Breast cancer is the 2nd most common cancer worldwide, with half a million women dying from the disease each year, ranking women the 2nd in death due to cancer and seriously threatening women's life health[1]. Neovascularization is closely related to the growth, infiltration and metastasis of breast cancer cells and within the interval of chemotherapy, the damaged tumor vasculature can undergo reconstruction, greatly reducing the efficacy and leading to a poor prognosis of breast cancer patients and therefore, the inhibition of angiogenesis is a key point in the treatment of breast cancer[2,3]. Phosphatase and Tensin Homology Deleted on Chromosome Ten (PTEN) is the only gene with dual phosphatase activity that has been shown to induce several malignancies, including breast and gastric cancer and can mediate tumor angiogenesis by regulating Protein kinase B (AKT) phosphorylation, inhibiting PTEN expression, enhancing AKT phosphorylation and alleviating hypoxic glucose deprivation injury in vascular endothelial cells, driving their survival and migration; up-regulation of PTEN expression can inhibit AKT phosphorylation, reduce gastric cancer tissue angiogenesis and inhibit cancer cell proliferation and metastasis[4-7]. Therefore, we hypothesized that PTEN/AKT signaling could be a potential target to inhibit the angiogenesis of transplanted tumors in nude mice with breast cancer cells. Formononetin, also known as formoononetin and neochanin, extracted from the legume red clover, has good anticancer activity and is an effective component of Astragalus membranaceus, which can induce apoptosis of human esophageal cancer cells and inhibit breast cancer cell proliferation and migration, but it is still unclear whether formononetin can regulate PTEN/AKT pathway expression and the angiogenesis process in transplanted tumor in nude mice with breast cancer cells[8,9]. In this study, a model of transplanted tumor in nude mice with breast cancer cells was established to carry out relevant studies.
Materials and Methods
Materials:
Experimental animals: Bagg and Albino (BALB)/C female nude mice, 5-6 w old, weighing 15-18 g, were purchased from the experimental animal center of Sun Yat-sen University and used the License No. SYXK (Y) 2017-0081 and the Production License No. SCXK (Y) 2016-0029; all mice were housed in the barrier environment animal room of our hospital with free access to food and water, at a temperature of 20°-26° and a humidity of 40 %-70 % and were acclimatized for 1 w before use in experiments.
Reagents and materials: Human breast cancer cells Michigan Cancer Foundation 7 (MCF 7) (Cat No. CM-H061), Shanghai Gaining Biotech Co., Ltd.; formononetin (Cat No. 485-72-3, content 99 %), Guangzhou Cafen Biotech Co., Ltd.; taxol (Cat No. Z161107), Chongqing Lummy Pharmaceutical Co., Ltd.; fetal bovine serum (Cat No. 7907456), Shanghai Hengyuan Biotechnology Co., Ltd. Dulbecco's Modified Eagle Medium (DMEM) (Cat No. 184723), Invitrogen Corp., United States of America (USA); trypsin- Ethylenediaminetetraacetic Acid (EDTA) digestion solution, penicillin streptomycin mixture (Cat No. T1320 and P1400), Beijing Solarbio Science and Technology Co., Ltd.; Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) apoptosis detection kit (Cat No. T2190), Shanghai Hengfei Bio Technology Co., Ltd.; rat two-step immunohistochemical kit, Diaminobenzidine (DAB) kit (Cat No. k137723c and ZLI-9017), Beijing Zhong Shan-Golden Bridge Biological Technology Co., Ltd.; primary rabbit Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), AKT and phospho-AKT antibodies, Immunoglobulin G (IgG)-Horseradish Peroxidase (HRP) secondary antibodies (Cat No. PA5-85082, 44-609G, 44-621G, A-11034), cell signaling technology, USA; primary rabbit PTEN, Cluster of Differentiation 31 (CD31) and Vascular Endothelial Growth Factor (VEGF) antibodies, rat VEGF Enzyme-Linked Immunosorbent Assay (ELISA) kit (Cat No. ab32199, ab119339, ab46154, ab100786), Abcam, USA; protein lysate, Bicinchoninic Acid (BCA) kit (Cat No. P0013K, P0011), Beyotime Biotech Inc., Shanghai and so on.
Instrument: Micro plate reader, protein electrophoresis apparatus and transmembrane apparatus (Models 680, 1659001, Trans-Blot SD), Bio-Rad, USA; light microscope (model SMZ745), Nikon Corporation, Japan; microtome (model CM3050S), manufactured by Leica, Germany; precision electronic scale, Sanyo Corporation, Japan; Gel imager (Model GIS-500), Miulab Corporation and so on.
Methods:
Preparation of animal model and group administration: The model of transplanted tumor in nude mice with breast cancer cells was prepared according to the literature[10,11], and the purchased cryopreserved cells were quickly thawed in a 39° water bath, centrifuged, resuspended in DMEM medium (10 % fetal bovine serum, 100 U/ml penicillin), cultured at 37° in a 5 % Carbon dioxide (CO2) cell incubator and passaged for 2-3 passages, MCF7 cells at logarithmic growth stage were taken. After digestion of trypsin, it is prepared as 1×107/ml cell suspension. Tumor bearing mice were established by inoculation under the fat pad of the left second nipple of mice at a dose of 0.2 ml per mouse, and a tumor mass with a diameter of ≥1 cm was considered to indicate successful model establishment. A total of 63 mice were created, with 60 successes and were randomly divided into a control group, a low dose (0.1 mg/g) group of formononetin, a middle dose (0.2 mg/g) group of formononetin, a high dose (0.4 mg/g) group of formononetin and a taxol (10 μg/g) group, with 12 mice in each group.
In reference to the literature, formononetin[12] and taxol[11] were dissolved in normal saline and prepared as 0.01, 0.02, 0.04 mg/μl formononetin solution, 1 μg/μl taxol solution, formononetin low dose group, formononetin middle dose group, formononetin high dose group mice were gavaged with 10 μl/g formononetin solution and 10 μl/g taxol solution of formononetin group mice were tail vein injected, mice in the control group were intragastrically administered with the same dose of normal saline, while the same dose of normal saline was injected via the tail vein and the administration time mentioned above was a total of 21 d.
Detection of transplanted tumor volume and specimen collection: After 24 h of completed administration, mice were killed by decapitation, 2 ml of blood was taken from abdominal aorta, supernatant was taken after centrifugation and serum was obtained and stored in liquid nitrogen for further use. Tumor masses were dissected and separated, and tumor volume (V) was calculated by measuring the tumor length (a) and short diameter (b) with a straight ruler, using the formula: V=a×b2. 0.5 g of transplanted tumor tissue was clipped and stored in liquid nitrogen for later use. The remaining tissues were fixed with paraformaldehyde (4 %), dehydrated by alcohol in accordance with the concentration gradient from low to high, transparent by xylene and embedded in paraffin. After the above operations were completed, the pathological tissues were made into routine pathological sections using microtome and stored for later use.
TUNEL staining detection of apoptosis in transplanted tumor cells: Each group was selected to save an alternate routine pathological section, which was deparaffinized first and then processed in accordance with the concentration gradient of alcohol from high to low, the processed pathological sections were taken for TUNEL staining and the staining was performed with the TUNEL apoptosis detection kit and the detection process was according to the instructions, followed by dehydration, transparency and sealing treatment. After sealing, the staining results were observed under a light microscope, the apoptotic cells were dark brown, the normal tumor cells were blue, five optional fields were photographed and the proportion of apoptotic cells was calculated with the formula;
Apoptotic cell ratio=[Apoptotic cells/(apoptotic cells+normal tumor cells)]×100 %
Immunohistochemical detection of angiogenesis: After deparaffinization and graded alcohol (high to low) treatment, were treated in 0.3 % Hydrogen peroxide (H2O2) for 30 min, then blocked in serum room temperature for 2 h, placed in rabbit CD31 primary antibody solution, incubated overnight at 4° in refrigerator, rinsed in distilled water and secondary antibodies were incubated using rat two step immunohistochemical kit, the operation procedure was based on the instructions. Distilled water was used for rinsing; DAB kit was used for full color development. After completion, they were rinsed again, dehydrated and cover slipped before the staining results were observed under a light microscope. CD31 positive cells, cell nuclei were blue, five optional fields were photographed and the proportion of CD31 positive cells was calculated according to the formula;
Proportion of CD31 positive cells=CD31 positive cells/tumor cells×100 %
Detection of serum VEGF levels: Select the spare serum, and test the VEGF level after thawing (in a refrigerator at 4°), the test kit is an ELISA kit and the test process is based on the instructions.
Western blot: The expression levels of VEGF proteins and proteins related to PTEN/AKT pathway were determined by Western blot. Part of tissues from the transplanted tumors was selected, cut and put into protein lysis solution (containing protease inhibitors) and then homogenized in a homogenizer, followed by centrifugation at 4° and the upper supernatant was collected after the completion of centrifugation, in which total protein was extracted and then the levels of total protein in the samples of each group were examined, the detection was performed using a BCA kit, operating strictly based on the instructions. Aliquots from each group containing equal amounts of protein were then selected and proteins were separated by electrophoresis. After separation, all proteins were placed on Poly Vinylidene Fluoride (PVDF) membranes, blocked for 2 h at room temperature (5 % nonfat milk), incubated overnight at 4° on ice with rabbit primary antibodies against PTEN, AKT, p-AKT and VEGF (1:1000), rinsed three times with Tris Buffered Saline Tween 20 (TBST) solution, mounted in IgG-HRP secondary antibody solution (1:2000), incubated for 2 h on a shaker, TBST solution was used for 3 rinses, developed in Electrochemiluminescence (ECL) and then photographed in a gel imager, and the protein bands were analyzed using Image Lab software to obtain the relative expression amounts of proteins in each group. All the above data were carefully recorded and collated.
Statistical processing:
Data were analyzed statistically using Statistical Package for the Social Sciences (SPSS) 20.0; the metrology data were expressed as the mean±standard deviation (x̄ ±s), one-way Analysis of Variance (ANOVA) was performed with Least Significant Difference (LSD)-t test for pairwise comparisons among multiple groups and p<0.05 was considered statistically significant.
Results and Discussion
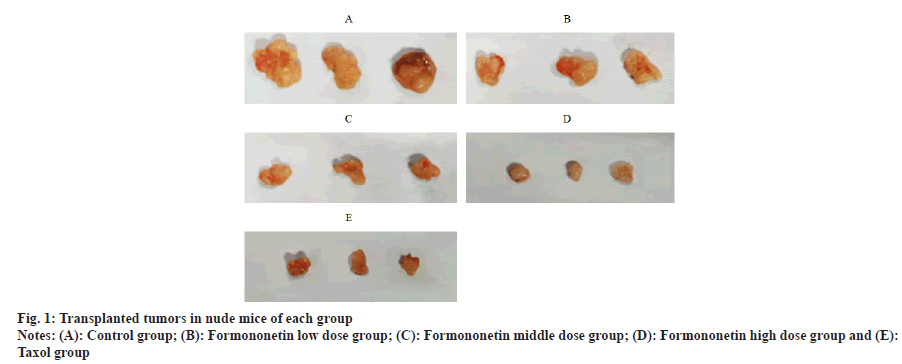
Compared with the control group, the volumes of transplanted tumors in nude mice treated with formononetin low, middle and high dose groups or taxol group were decreased in a dose-dependent manner (p<0.05); there was no difference between taxol group and high dosage group of formononetin (p>0.05) as shown in fig. 1 and Table 1.
| Group | Transplanted tumor volume (mm3) |
|---|---|
| Control group | 1520.03±210.82 |
| Formononetin low dose group | 1132.85±203.11a |
| Formononetin middle dose group | 815.13±132.38ab |
| Formononetin high dose group | 420.65±80.56abc |
| Taxol group | 404.67±76.12abc |
Note: ap<0.05 vs. control group; bp<0.05 vs. formononetin low dose group and cp<0.05 vs. formononetin middle dose group
Table 1: Comparison of Transplanted Tumor Volumes among Groups of Nude Mice (x̄±s, n=12)
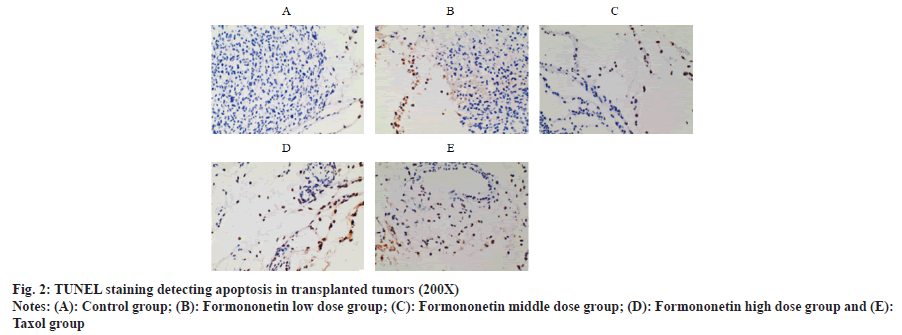
Compared with the control group, the proportion of apoptotic cells of transplanted tumors in nude mice treated with formononetin low, middle and high dose groups or taxol group were increased in a dose-dependent manner (p<0.05); there was no difference between taxol group and high dosage group of formononetin (p>0.05) as shown in fig. 2 and Table 2.
| Group | Proportion (%) of apoptotic cells |
|---|---|
| Control group | 1.68±0.27 |
| Formononetin low dose group | 32.58±5.01a |
| Formononetin middle dose group | 57.32±12.37ab |
| Formononetin high dose group | 74.63±18.16abc |
| Taxol group | 73.17±19.02abc |
Note: ap<0.05 vs. control group; bp<0.05 vs. formononetin low dose group and cp<0.05 vs. formononetin middle dose group
Table 2: Comparison of the Proportion of Apoptotic Cells in the Transplanted Tumors of Nude Mice in Each Group (x̄±s, n=12)
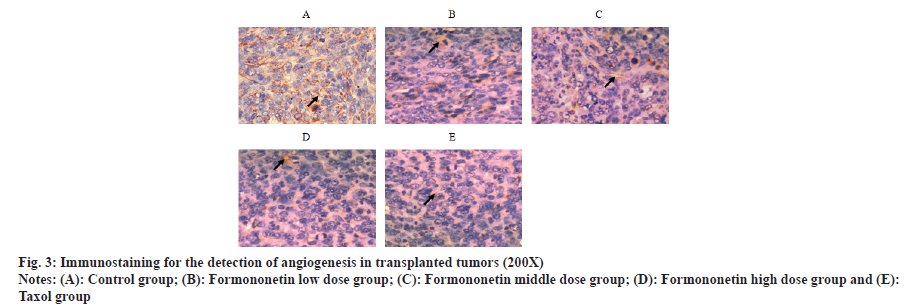
Compared with the control group, the proportion of CD31 positive cells of transplanted tumors in nude mice treated with formononetin low, middle and high dose groups or taxol group were decreased in a dose-dependent manner (p<0.05); there was no difference between taxol group and high dosage group of formononetin (p>0.05) as shown in fig. 3 and Table 3.
| Group | Proportion (%) of CD31 positive cells |
|---|---|
| Control group | 73.65±16.07 |
| Formononetin low dose group | 50.81±10.02a |
| Formononetin middle dose group | 31.12±5.71ab |
| Formononetin high dose group | 11.93±2.13abc |
| Taxol group | 10.12±2.05abc |
Note: ap<0.05 vs. control group; bp<0.05 vs. formononetin low dose group and cp<0.05 vs. formononetin middle dose group
Table 3: Comparison of the Proportion of CD31 Positive Cells in the Transplanted Tumors of Nude Mice in Each Group (x̄±s, n=12)
Compared with the control group, the levels of VEGF in the serum in nude mice treated with formononetin low, middle and high dose groups or taxol group were decreased in a dose-dependent manner (p<0.05); there was no difference between taxol group and high dosage group of formononetin (p>0.05) as shown in Table 4.
| Group | Serum VEGF levels (ng/l) |
|---|---|
| Control group | 51.86±6.32 |
| Formononetin low dose group | 41.13±4.71a |
| Formononetin middle dose group | 30.67±2.85ab |
| Formononetin high dose group | 18.32±1.63abc |
| Taxol group | 16.93±1.80abc |
Note: ap<0.05 vs. control group; bp<0.05 vs. formononetin low dose group and cp<0.05 vs. formononetin middle dose group
Table 4: Comparison of Serum VEGF in Each Group (x̄±s, n=12)
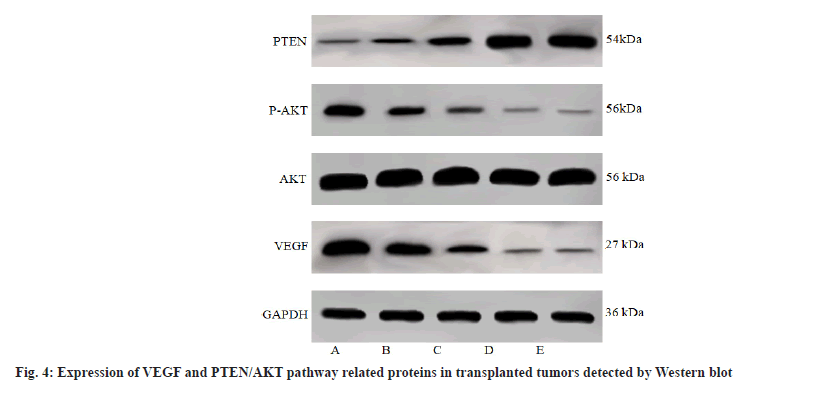
When compared with the control group, the expression of VEGF and the p-AKT/AKT, and PTEN expression of the transplanted tumors in nude mice treated with low, middle and high doses of formononetin or taxol were decreased and all showed an increase in PTEN expression in a dose dependent manner (p<0.05); there was no difference between taxol group and formononetin high dose group (p>0.05) as shown in fig. 4 and Table 5.
| Group | VEGF/GAPDH | PTEN/GAPDH | p-AKT/AKT |
|---|---|---|---|
| Control group | 1.89±0.37 | 0.24±0.05 | 0.72±0.13 |
| Formononetin low dose group | 0.98±0.21a | 0.43±0.08a | 0.45±0.08a |
| Formononetin middle dose group | 0.47±0.09ab | 0.92±0.17ab | 0.27±0.05ab |
| Formononetin high dose group | 0.10±0.02abc | 1.73±0.32abc | 0.08±0.02abc |
| Taxol group | 0.13±0.03abc | 1.76±0.36abc | 0.07±0.01abc |
Note: ap<0.05 vs. control group; bp<0.05 vs. formononetin low dose group and cp<0.05 vs. formononetin middle dose group
Table 5: Comparison of VEGF and PTEN/AKT Pathway Related Protein Expression in Transplanted Tumor of Nude Mice in Each Group (x̄±s, n=12)
The treatment of breast cancer is mainly based on surgery followed by chemoradiotherapy, combined with comprehensive measures of endocrine and molecular targeted therapy, but the clinical treatment results are not good, especially for advanced breast cancer .
Breast cancer develops hematogenous metastasis early and tumor recurrence and drug resistance due to angiogenesis during treatment are the main reasons for its poor efficacy[13]. Cancer cell invasion, metastasis and growth need to rely on neovascularization. Studies have found that VEGF is the main cytokine that prompts blood vessel growth, can bind to receptors, induce endothelial cell proliferation, migration and form new vascular structures in and around tumor tissue, providing nutrients required for tumor growth[14]. CD31 is a platelet-endothelial cell adhesion molecule expressed in the interendothelial tight junctions, which can participate in mediating angiogenesis and is a marker for neovascularization[15]. Formononetin is a flavonoid with strong anti-tumour activity, which can inhibit the proliferation and induce apoptosis of colorectal cancer cells[16] and can inhibit the proliferation of prostate cancer cells by downregulating the expression of cell cycle related proteins[17], and has a better effect on different subtypes of breast cancer[18], but the effect of formononetin on breast cancer angiogenesis has not been investigated so far. In this paper, our results showed that treatment of transplanted tumor in nude mice with breast cancer cell with formononetin reduced the transplanted tumor volume in nude mice, reduced the proportion of apoptotic cells, the proportion of CD31 positive cells, the serum VEGF level and VEGF protein expression in the transplanted tumors in a dosedependent manner, indicating that formononetin inhibited the growth and VEGF expression of transplanted tumors of breast cancer cells, prevented angiogenesis and enhanced the effect with increasing doses.
PTEN is a tumor suppressor gene located on human chromosome 10, which has dual activities of lipid phosphatase and protein phosphatase and is involved in regulating the formation and disease progression of a variety of malignancies, known as the "gatekeeper" of tumor formation growth by regulating the phosphorylation of AKT. Up-regulation of PTEN expression, can inhibit AKT phosphorylation and then inhibit liver cancer tumor growth and angiogenesis, and overexpression of PTEN can inhibit breast cancer cell MCF7 proliferation and induce their apoptosis, therefore, PTEN/AKT signaling may be a key functional target to inhibit tumor growth and angiogenesis of transplanted tumor in nude mice with breast cancer cells[19,20]. However, whether formononetin can modulate PTEN/ AKT pathway expression in transplanted tumor in nude mice with breast cancer cells is still unknown. The results showed that PTEN protein expression was decreased and p-AKT/AKT was increased in the transplanted tumors of nude mice with breast cancer transplanted tumor, indicating that the PTEN/AKT pathway was in an activated state during the growth of the transplanted tumors and may be involved in mediating tumor vascularization in breast cancer cell transplanted tumors. After treatment with formononetin, the protein expression of PTEN increased, p-AKT/ AKT decreased and showed dose-dependent manner, indicating that formononetin could enhance the protein expression of PTEN and inhibit the phosphorylation of AKT, we speculated that formononetin might act on tumor growth and angiogenesis by inhibiting the activation of PTEN/AKT pathway, which was enhanced with higher dosage.
In summary, formononetin can up-regulate PTEN protein expression, down-regulate AKT phosphorylation levels, inhibit the growth of transplanted tumors of nude mice with breast cancer, prevent angiogenesis, induce apoptosis of breast cancer cells and ultimately reduce the tumor volume, which provides ideas for the clinical treatment of breast cancer and regulation of PTEN/AKT signaling may be part of its pharmacological mechanism, but this study only preliminarily confirms that the use of formononetin in the treatment of breast cancer can promote PTEN expression, reduce AKT phosphorylation levels, without up or down-regulation of PTEN expression for control validation. Evidence about the pharmacological mechanism is insufficient and further in-depth studies are needed.
Funding:
This work was supported by Zhejiang health science and technology project (2023KY1030).
Conflicts of interest:
The authors declared no conflict of interests.
References
- Pavuluri S, Sharp JA, Lefevre C, Nicholas KR. The effect of mammary extracellular matrix in controlling oral and mammary cancer cells. Asian Pac J Cancer Prev 2018;19(1):57.
[Crossref] [Google Scholar] [PubMed]
- van Loevezijn AA, Bartels SA, van Duijnhoven FH, Heemsbergen WD, Bosma SC, Elkhuizen PH, et al. Internal mammary chain sentinel nodes in early-stage breast cancer patients: Toward selective removal. Ann Surg Oncol 2019;26(4):945-53.
[Crossref] [Google Scholar] [PubMed]
- Liang H, Xiao J, Zhou Z, Wu J, Ge F, Li Z, et al. Hypoxia induces miR-153 through the IRE1α-XBP1 pathway to fine tune the HIF1α/VEGFA axis in breast cancer angiogenesis. Oncogene 2018;37(15):1961-75.
[Crossref] [Google Scholar] [PubMed]
- Li X, Zhang R, Liu Z, Li S, Xu H. The genetic variants in the PTEN/PI3K/AKT pathway predict susceptibility and CE (A) F chemotherapy response to breast cancer and clinical outcomes. Oncotarget 2017;8(12):20252.
[Crossref] [Google Scholar] [PubMed]
- Al-Dhfyan A, Alhoshani A, Korashy HM. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation. Mol Cancer 2017;16(1):14-31.
[Crossref] [Google Scholar] [PubMed]
- Xue L, Huang J, Zhang T, Wang X, Fu J, Geng Z, et al. PTEN inhibition enhances angiogenesis in an in vitro model of ischemic injury by promoting Akt phosphorylation and subsequent hypoxia inducible factor-1α up regulation. Metab Brain Dis 2018;33(5):1679-88.
[Crossref] [Google Scholar] [PubMed]
- Wu ZH, Lin C, Liu CC, Jiang WW, Huang MZ, Liu X, et al. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res Commun 2018;501(4):1068-73.
[Crossref] [Google Scholar] [PubMed]
- Liu Y. Effect of active components in Astragalus membranaceus on apoptosis of human esophageal carcinoma HCE-4 cells. Drug Eval Res 2016;39(3):377-81.
- Ge X, Li L, Wang F. Formononetin inhibiting HIF-1α/CXCR4 signaling in breast cancer cells and cell proliferation and migration. Henan Med Res 2016;25(9):1542-5.
- Wang F, Zhu J, Yang H. Correlation study between RSK4 and Ki-67, cyclin D1, CXCR4 and E-cadherin in transplanted tumor of nude mice with breast cancer. Chin J Oncol Prev Treat 2018;10(2):136-40.
- Li J, Huang W, Liu B. Orthotropic transplantation model of breast cancer cells and efficacy of a combination of taxol and epirubicin. Chin Trop Med 2018;18(8):766-9.
- Zhang L, Ren Z, Xue Y. Effect of formononetin on endostatin, VEGF, MMP-2, bFGF and tumor markers in pleural fluid and serum of elderly patients with advanced lung cancer. Chin J Biochem Pharm 2015;35(8):160-3.
- Qingqing H, Jian Z, Dayong Z, Ziyi F, Luming Z, Peng Z, et al. Robot-assisted internal mammary lymph node chain dissection for breast cancer. Clin Breast Cancer 2018;18(4):e441-5.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Luan Y, Miao X, Sun C, Li K, Huang Z, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF–integrin cooperative signaling. Br J Cancer 2017;117(5):695-703.
[Crossref] [Google Scholar] [PubMed]
- Xing J, He W, Ding YW, Li Y, Li YD. Correlation between contrast-enhanced ultrasound and micro vessel density via CD31 and CD34 in a rabbit VX2 lung peripheral tumor model. Med Ultrason 2018;20(1):37-42.
[Crossref] [Google Scholar] [PubMed]
- Li T, Li X, Gao F. Effect and mechanism of formononetin on proliferation and apoptosis of colorectal cancer cells. Shandong Med J 2016;56(10):1-3.
- Zhao X, Chen J. Formononetin inhibiting prostate cancer PC-3 cell proliferation and the underlying mechanism. Acta Med Sin 2016;29 (1):6-9.
- Sheng J, Chen H. Effect of formononetin combined with MK2206 on cell proliferation and apoptosis in different subtypes of breast cancer. Chin J Cancer Prev Treat 2015;22(13):12-17.
- Wu D, Li M, Tian W, Wang S, Cui L, Li H, et al. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci Rep 2017;7(1):5134.
[Crossref] [Google Scholar] [PubMed]
- Liu T, Guo J, Zhang X. MiR-202-5p/PTEN mediates doxorubicin-resistance of breast cancer cells via PI3K/Akt signaling pathway. Cancer Biol Ther 2019;20(7):989-98.
[Crossref] [Google Scholar] [PubMed]