- *Corresponding Author:
- Shuang Jiang
Department of Ophthalmology, The Third Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province 121000, China
E-mail: joa810121@163.com
| Date of Received | 27 November 2021 |
| Date of Revision | 09 November 2022 |
| Date of Acceptance | 13 March 2023 |
| Indian J Pharm Sci 2023;85(2):310-317 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to investigate the effects of flavonoid glycoside on mitochondrial dysfunction and inflammatory oxidative stress in macular degeneration. Sixty specific-pathogen-free male Bagg and Albino mice were randomly divided into control, model and flavonoid glycoside. Human retinal pigment epithelium cells ARPE-19 were cultured and treated with dimethyl sulfoxide, sodium iodate and flavonoid glycoside respectively. Fundus fluorescein angiography was used to detect the number of mouse retinal leakage; optical coherence tomography retinal thickness. A wave and B wave were detected by electroretinogram. Hematoxylin and eosin staining was used to observe the morphology of mouse retina; phalloidin, translocase of the outer mitochondrial membrane complex subunit 20 and cyclooxygenase III staining were performed on mouse retinal pigment epithelium cells by immunofluorescence. Adenosine triphosphate content in retinal pigment epithelium cells of mice in each group was detected. Compared with the control mice, the number of retinal leakage points in the model increased, the retinal thickness, A wave and B wave decreased, and the levels of Fas and FasL protein increased significantly. After treatment with flavonoid glycoside, the number of retinal leakage decreased the retinal thickness, A wave and B wave increased, and the levels of Fas and FasL protein decreased significantly. Flavonoid glycoside can significantly improve the mitochondrial dysfunction and inflammatory oxidative stress of retinal pigment epithelium cells and play a good role in protecting macular degeneration.
Keywords
Flavonoid glycoside, macular degeneration, mitochondrion, oxidative stress
Macular degeneration is a major blinding eye disease in people over 65 y old, which can lead to irreversible vision loss. At present, the global prevalence rate is about 3 %. Macular degeneration is an age-related blindness degenerative fundus disease. With the aging of the population, the number of patients increases year by year. The pathogenesis of macular degeneration is complex. Retinal Pigment Epithelium (RPE) degeneration causes the degeneration of retinal receptor cells, which is a factor leading to visual impairment. The mechanisms that affect RPE degeneration may include inflammation, oxidative stress and mitochondrial dysfunction [1-5].
Sodium iodate (NaIO3) is a strong oxidant, which can be used as a retinal toxin to cause RPE damage, so it can be used to induce macular degeneration. NaIO3 can be used to construct mouse macular degeneration model and in vitro macular degeneration model.
Macular degeneration can be divided into two types; dry and wet, of which 90 % are dry. The fundus of dry macular degeneration is mainly manifested as atrophy of choroidal capillaries, vitreous verruca and RPE. In the late stage, map atrophy of the macular area can occur, leading to a significant decline in vision. Some dry macular degeneration will progress to wet macular degeneration. In recent years, great progress has been made in the treatment of wet macular degeneration due to Anti-Vascular Endothelial Growth Factor (VEGF), but there is no effective treatment for dry macular degeneration. Flavonoid glycoside is the main active components in Ginkgo biloba leaves. Many studies have confirmed that flavonoid glycoside have the pharmacological functions of antioxidation, improving brain cell metabolism, analgesia, anti-inflammatory, bacteriostasis, anti-cancer and enhancing immunity. Studies have shown that flavonoid glycoside have a good effect on scavenging free radicals, superoxide anion, hydrogen peroxide and other harmful substances. However, whether flavonoid glycoside can alleviate macular degeneration has not been reported [6-12].
The aim of this study was to explore the effects of flavonoid glycoside on mitochondrial dysfunction and inflammatory oxidative stress in macular degeneration by establishing a mouse model of macular degeneration and an in vitro cell model.
Materials and Methods
General information:
60 Specific-Pathogen-Free (SPF) grade 7 w old male Bagg and Albino (BALB/c) mice were purchased from Hubei Beiente Biotechnology Co., Ltd. with the license number of SCXK (E) 2021-0027. The average body weight of mice was (20.42±2.94) g. Feeding conditions were; temperature 25°, humidity 55 %, natural light, free drinking and eating. All experimental processes conform to the 3R principle.
Construction and treatment of macular degeneration mouse model:
Prior to the experiment, no eye abnormalities were found in all the mice under the slit lamp. Sixty rats were randomly divided into control, model and flavonoid glycoside with 20 mice each. NaIO3 powder is dissolved in normal saline to form a 20 g/l solution, which is temporarily stored in a refrigerator at 4°. Mice in each group except the control were injected via tail vein 25 μl/g NaIO3, after the injection, place the mice in a quiet, clean and warm environment until they wake up for subsequent experiments.
The control mice were injected with the same volume of normal saline through the tail vein. After the mice woke up, the flavonoid glycoside was given 20 and 40 mg/kg flavonoid glycoside by gavage and the flavonoid glycoside were dissolved in normal saline to prepare a fixed 1 ml solution for gavage; the control and model were perfused with equal volume of normal saline once a day for 1w.
Cell culture and treatment:
Human RPE (ARPE-19) cells were purchased from ATCC library and further verified by Shanghai Shengyi Applied Biotechnology Co., Ltd. through Short Tandem Repeat (STR) analysis. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F12 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 10 % Fetal Bovine Serum (FBS) at 37° and 5 % Carbon dioxide (CO2) was added in the incubator.
Before the experiment, change the culture medium every 2 d. For each experiment using cultured ARPE- 19 cells, the culture density was 1×104 cells in 96 well plates, 2×104 cells in 24 well plate, 2×105 cells in 6 well plate, 5×105 cells in 6 cm plates. 1 d later, ARPE-19 cells were treated with Dimethyl Sulfoxide (DMSO), NaIO3 (dissolved in DMSO) and flavonoid glycoside (dissolved in DMSO) for 24 h.
Fundus fluorescence contrast instrument detection:
The mouse mydriasis, 10 % sodium fluorescein injection (2 ml/kg) was injected intraperitoneally and after the sodium fluorescein was circulated to the fundus, the Fundus Fluorescein Angiography (FFA) was performed on the right eye of the mouse with the FFA instrument; after FFA examination, Optical Coherence Tomography (OCT) was performed and fundus photography was performed. The retinal thickness was measured by two skilled ophthalmologists.
Electroretinogram (ERG):
The mice were anesthetized with 1.0 % pentobarbital sodium buffer for 12 h after dark adaptation. After pupil dilation, the ERG was recorded with silver chloride click ring. The stainless steel needle behind the ear and tail was used as reference electrode and grounding electrode. After the baseline was stable, the ERG was detected.
Hematoxylin and Eosin (H&E) staining:
After fixing the eyeball with 4 % Paraformaldehyde (PFA), the eyeball was fixed and embedded with paraffin and the eyeball was sliced continuously parallel to the eye axis, with a thickness of 5 μm. Retinal sections were stained with hematoxylin, washed with distilled water and restained with eosin dye. After xylene transparent, neutral gum film, optical microscope (200×) used to observe the retinal structure.
Immunofluorescence:
After the mice were killed by decapitation, eyes were removed, 4 % PFA was fixed at room temperature for 1 h, cornea, lens, muscle and retina were removed and RPE was obtained. Fix RPE at 4 % PFA room temperature for 1 h, 1× Phosphate Buffered Saline (PBS) was washed for 5 min, followed by 1 h of chalk in PBS containing 10 % FBS and 0.5 % Triton X-100. Using Alexa Fluor® 594 phalloidin was used to stain RPE. After 30 min of staining, Translocase of the Outer Mitochondrial Membrane complex subunit 20 (TOMM20) was stained for 30 min; or Cyclooxygenase (COX) III staining for 30 min, 4',6-Diamidino-2- Phenylindole (DAPI) staining for 30 min and 1× PBS wash for 5 min.
Adenosine Triphosphate (ATP) determination:
ATP in RPE was measured using a fluorescent ATP assay kit. In short, RPE was dissected and quickly frozen in liquid nitrogen. After dissecting all the eyes, thaw the tissues with 2 ml Dounce homogenizer and 25 μl determine the homogenate in buffer solution. Centrifuge the sample at 4° at 13 000 g for 5 min. The supernatant was collected and stored on ice. Mix the sample or known standard with ATP reaction mixture or background control mixture into two parts and incubate them at room temperature for 30 min.
Detection of Malondialdehyde (MDA), Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH- Px) and Catalase (CAT) activities in mouse retina and RPE cells:
The retina of eyeball mice was extracted and homogenized, centrifuged at 3000 rpm at 4° for 10 min and the supernatant was used for detection. ARPE-19 cells were collected for detection. Sampling during testing was done. The Optical Density (OD) values were measured at the wavelengths of 550, 412 and 405 nm respectively according to the instructions of the MDA, SOD, CAT and GSH-Px test kits and the enzyme activity was calculated.
Western blot:
The Radio-Immunoprecipitation Assay (RIPA) lysate (BB-3209, Bebo Bio, Shanghai, China) was used to lyse and extract the total protein of the cell to be tested. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) separation, constant voltage 80 V electric transfer to Polyvinylidene Fluoride (PVDF) imprinted membrane. After the membrane sealing solution is sealed for 1 h, add monoclonal antibodies against Fas, FasL, Glutathione Peroxidase 4 (GPX4), NADPH Oxidase 4 (NOX4), Neurofibromin 2 (NF2), caspase 3, caspase 7, caspase 8, Tumor Necrosis Factor Receptor Superfamily Member 1 alpha (TNFRSF1α), Receptor-Interacting Protein Kinase (RIPK)-1, RIPK3 and incubate overnight at 4°. The second antibody of goat anti-rabbit Immunoglobulin G (IgG) (1:1000, Wuhan Bode Company, Wuhan, China) labeled with horseradish peroxidase was incubated at 37° for 1 h. PBS was washed for 3 times at room temperature, 5 min/time and color was developed.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 26.0 statistical software was used for data analysis. The measurement data was expressed as "x±s", the one way Analysis of Variance (ANOVA) was used for data analysis. p<0.05 indicates that the difference is statistically significant.
Results and Discussion
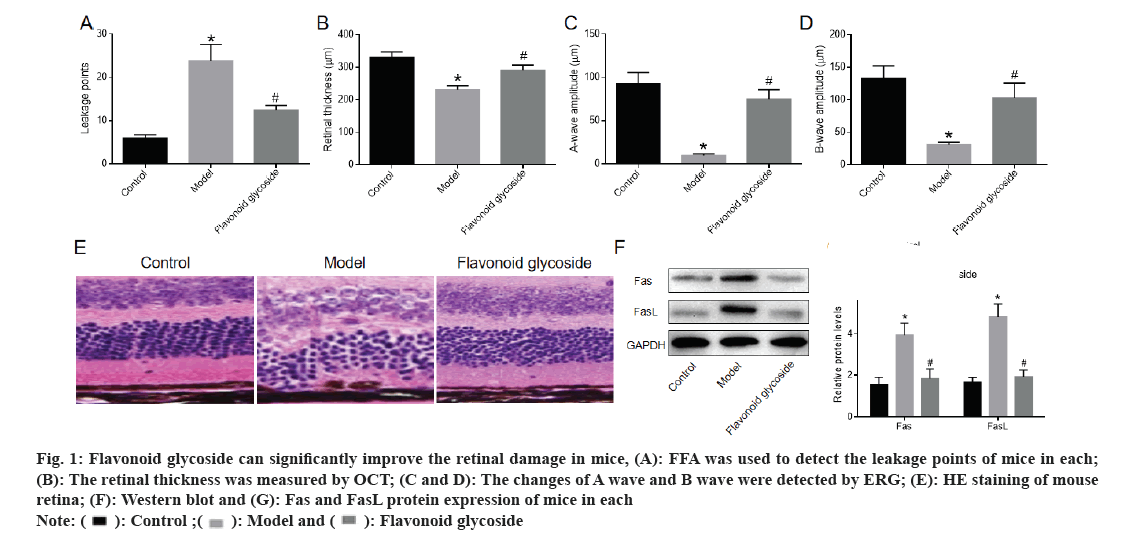
FFA detected the number of leakage points in the mouse retina. The number of leakage points in the model was significantly higher than that in the control. Compared with the model, the number of retinal leakage points in the flavonoid glycoside was significantly reduced (fig. 1A). OCT showed that the retinal thickness of mice in the model was significantly lower than that of mice in the control and the retinal thickness of mice after flavonoid glycoside treatment was significantly increased (fig. 1B). The amplitudes of A and B waves were recorded by ERG. The A and B waves of model mice were significantly lower than those of control mice. The A wave and B wave of mice treated with flavonoid glycoside were significantly higher than those of mice in the model (fig. 1C and fig. 1D). The retina of mice in each was stained with H&E and the cells in each layer of the retina of the control mice were arranged neatly and tightly; the cells in each layer of the retina of the model mice were in disorder, the outer nuclear layer was loosely arranged, the cells were embedded in the inner/outer segments of Inner Segment (IS)/Outer Segment (OS), the Inner Nuclear Layer (INL) cells in the inner layer became larger and loosely arranged, the amount of RPE deposits increased and the cells appeared vacuole. The cells in each layer of the flavonoid glycoside mice were gradually arranged in order, the Outer Nuclear Layer (ONL) and INL cells in the outer nuclear layer were closely arranged and the amount of RPE deposits was reduced (fig. 1E). The protein expression of Fas and FasL in the retina of model mice was significantly higher than that of control mice, while protein expression of Fas and FasL in retina of flavonoid glycoside mice was significantly lower than that of model mice (fig. 1F and fig. 1G). These results indicated that the flavonoid glycoside could significantly improve retinal damage in mice with macular degeneration.
Fig 1: Flavonoid glycoside can significantly improve the retinal damage in mice, (A): FFA was used to detect the leakage points of mice in each; (B): The retinal thickness was measured by OCT; (C and D): The changes of A wave and B wave were detected by ERG; (E): HE staining of mouse retina; (F): Western blot and (G): Fas and FasL protein expression of mice in each
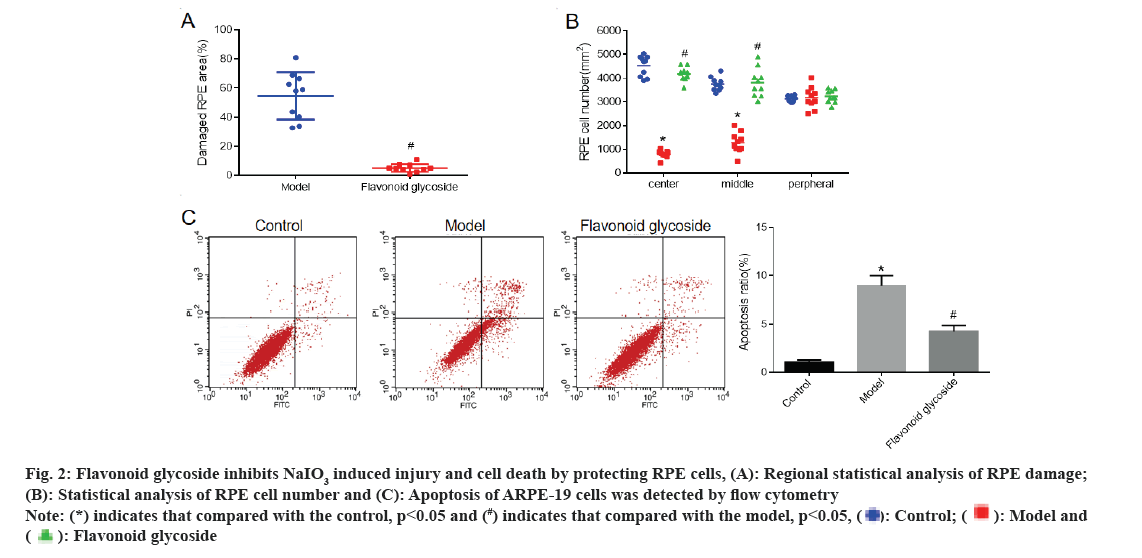
The RPE of mice was stained with phalloidin and the RPE damage area (fig. 2A) and RPE cell number (fig. 2B) of mice in each were detected respectively. The results showed that compared with the control mice, the number of RPE cells in the model mice was significantly reduced. Compared with model mice, RPE injury in flavonoid glycoside mice was significantly reduced and the number of RPE cells was significantly increased. To further investigate the effect of flavonoid glycoside on RPE cells, we cultured ARPE-19 human RPE cells and detected cell apoptosis by flow cytometry (fig. 2C). The results showed that the apoptotic rate of ARPE-19 (model) cells treated with NaIO3 was significantly increased compared to the control. Compared with NaIO3, the apoptotic rate of ARPE-19 cells treated with NaIO3 and flavonoid glycoside was significantly reduced.
Fig 2: Flavonoid glycoside inhibits NaIO3 induced injury and cell death by protecting RPE cells, (A): Regional statistical analysis of RPE damage; (B): Statistical analysis of RPE cell number and (C): Apoptosis of ARPE-19 cells was detected by flow cytometry Note: (*) indicates that compared with the control, p<0.05 and (#) indicates that compared with the model, p<0.05,

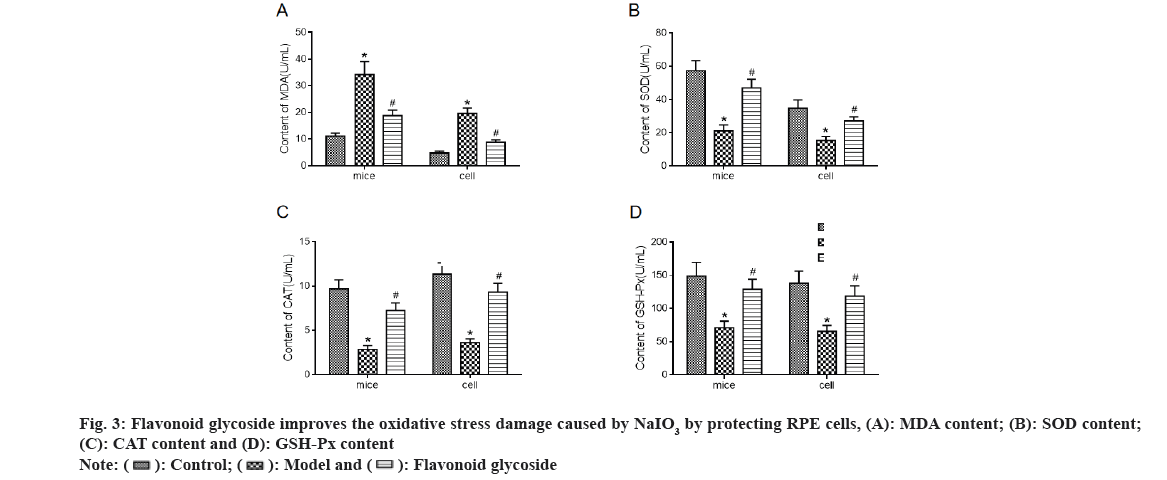
Levels of oxidative stress indicators MDA (fig. 3A), SOD (fig. 3B), CAT (fig. 3C) and GSH-Px (fig. 3D) in mouse retina and ARPE-19 cells were detected. The results showed that the MDA level in the model mice and cells increased significantly compared to the control, while the SOD, CAT and GSH-Px levels decreased significantly. Compared to the model, MDA levels in mice and cell flavonoid glycoside decreased significantly, while SOD, CAT and GSH- Px levels increased significantly.
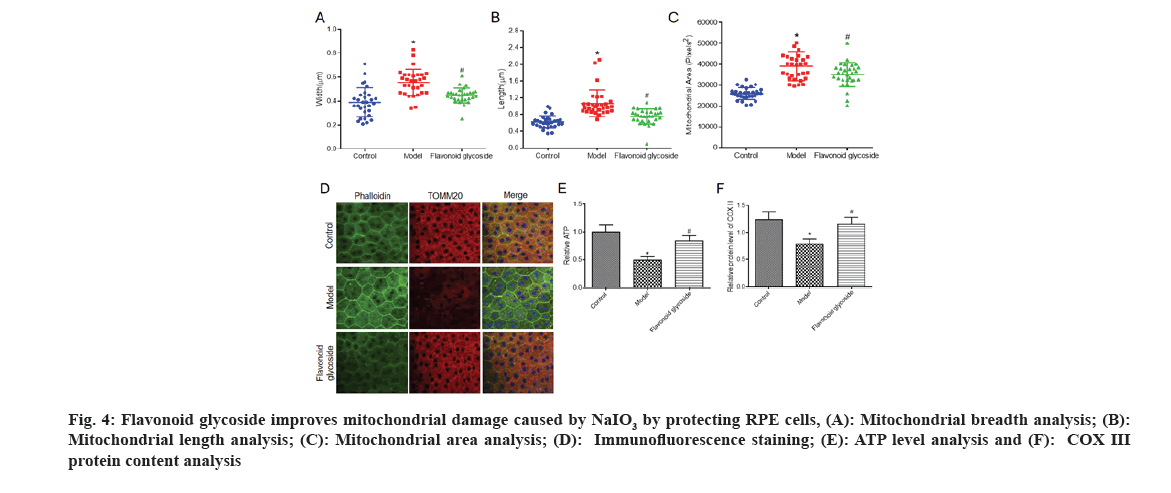
To further explore the protective mechanism of flavonoid glycoside on RPE cells, we observed the morphology of mitochondria in each of mice through transmission electron microscopy, and analyzed the width (fig. 4A), length (fig. 4B) and area (fig. 4C) of mitochondria. We found that the width, length and area of mitochondria in the model were significantly higher than those in the control. The morphology of mitochondria in flavonoid glycoside mice was significantly improved. We labeled F-actin with phalloidin to visualize RPE cell morphology and labeled the mitochondrial protein TOMM20.
Fig 4: Flavonoid glycoside improves mitochondrial damage caused by NaIO3 by protecting RPE cells, (A): Mitochondrial breadth analysis; (B): Mitochondrial length analysis; (C): Mitochondrial area analysis; (D): Immunofluorescence staining; (E): ATP level analysis and (F): COX III protein content analysis
The results showed that TOMM20 staining in the control mice was equal in the RPE cells, while in the mouse model it was reduced and the staining was inconsistent. The staining of TOMM20 in flavonoid glycoside mice increased significantly and was more uniform than that in model mice (fig. 4D). To further explore changes in mitochondrial function in mouse RPE cells in each, we measured ATP levels in the RPE cells of the three groups of mice. The ATP level of model mice was clearly reduced than the control mice; however the flavonoid glycoside mice were clearly increased. The results of the COX III protein content test showed that (fig. 4E), the COX III protein content of the model mice was significantly reduced compared to control mice. Compared with the mouse model, the protein content of COX III in the flavonoid glycoside mice was significantly increased.
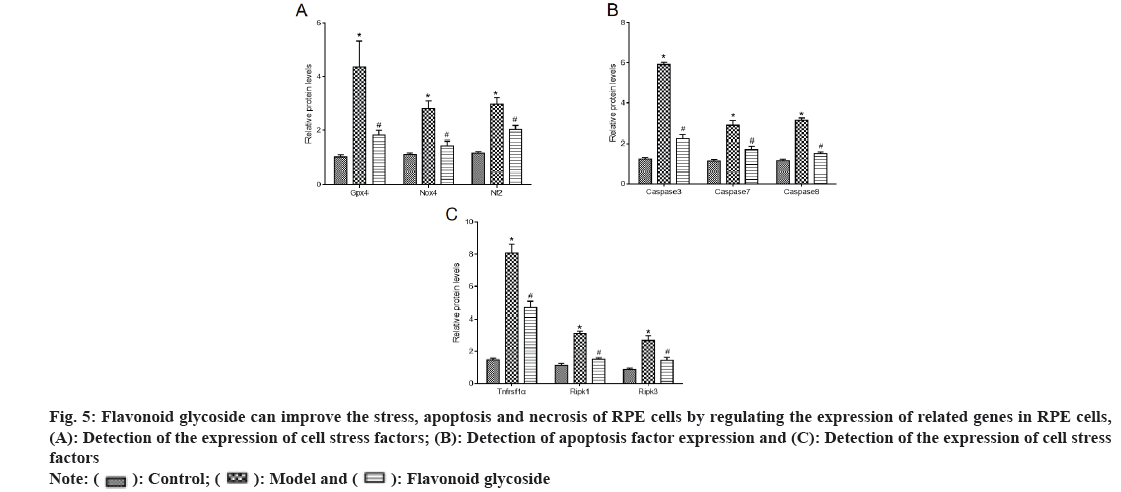
We detected the expression of stress (fig. 5A), apoptosis (fig. 5B) and necrosis (fig. 5C) related factors in mouse RPE cells and ARPE-19 cells. The results showed that, compared with the control, the stress factors GPX4, NOX4, NF2 proteins, apoptosis indicators caspase 3, caspase 7, caspase 8 and necrosis indicators TNFRSF1α, RIPK1, RIPK3 proteins in the model mice and cells were significantly increased. Compared with model mice and cells, the stress factors GPX4, NOX4, NF2 proteins, apoptosis indicators caspase 3, caspase 7, caspase 8 and necrosis indicators TNFRSF1α, RIPK1, RIPK3 proteins in flavonoid glycoside mice and cells were significantly reduced.
Fig 5: Flavonoid glycoside can improve the stress, apoptosis and necrosis of RPE cells by regulating the expression of related genes in RPE cells, (A): Detection of the expression of cell stress factors; (B): Detection of apoptosis factor expression and (C): Detection of the expression of cell stress factors
The biggest harm of macular degeneration is the permanent loss of vision in the central macular region, which has become the second disease of eye diseases in people over 50 y old in China. Wet macular degeneration is related to abnormal choroidal neovascularization. At present, the commonly used treatment method is intravitreal injection of VEGF antibody, which can achieve the purpose of treatment by inhibiting angiogenesis and the effect is good. Dry macular degeneration is mainly characterized by visual damage caused by massive destruction of RPE, which plays an important role in maintaining the blood retinal barrier, participating in the visual circulation and phagocytosis of exfoliated cells[13-18]. The destruction of RPE can lead to the aggravation of inflammatory reaction and oxidative stress. NaIO3 can specifically act on RPE, resulting in the destruction of RPE barrier and photoreceptor cell functions, thus causing pathological changes in retinal photoreceptors and choroids, resulting in retinal damage. Therefore, reducing oxidative damage is of great significance for disease treatment.
Studies have shown that flavonoid glycoside can effectively scavenge DPPH free radicals and animal experiments have shown that they have strong reducing power and can effectively inhibit the production of MDA, a lipid peroxidation product. Some studies have reported the antioxidant effect of flavonoid glycoside in vitro. The results show that flavonoid glycoside can better inhibit the autoxidation and induced oxidation of mice liver homogenate and have a better scavenging effect on a variety of free radicals[19-22]. In addition, other studies have confirmed that flavonoid glycoside have anti-inflammatory and analgesic effects. It has been reported that flavonoid glycoside can significantly inhibit ear swelling and paw swelling in mice, so it is concluded that flavonoid glycoside have certain anti- inflammatory effects. In this study, a mouse model of macular degeneration was established and the number of retinal leakage, retinal thickness, A wave and B wave, and retinal apoptosis indicators Fas and FasL were compared[23-25]. We found that flavonoid glycoside can significantly reduce the number of retinal leakage, improve the retinal thickness and increase the retinal A wave and B wave. These results indicate that flavonoid glycoside have a very significant effect on the recovery of retinal injury[26-30].
Through further exploration, we found that the improvement of flavonoid glycoside on macular degeneration may be achieved by acting on RPE cells. In the mouse experiment, it was found that the RPE damage area of flavonoid glycoside mice was significantly reduced and the number of RPE cells was significantly increased[31-33]. Cell experiments showed that flavonoid glycoside could significantly reduce the proportion of apoptosis. These results suggest that flavonoid glycoside can improve macular degeneration by protecting RPE cells from damage.
In the in-depth mechanism exploration, we found that flavonoid glycoside may protect RPE cells from damage by improving the stress and mitochondrial dysfunction of RPE cells. The results of this study showed that flavonoid glycoside could significantly reduce the content of MDA in the retina and RPE cells of macular degeneration mice and significantly increase the content of SOD, CAT and GSH-Px. Mitochondrial dysfunction in RPE cells may lead to the disruption of the metabolic balance of RPE cells[34]. The results of this study showed that the mitochondria of the mice with macular degeneration showed obvious swelling, the width, length and area of the mitochondria were significantly increased and the ATP level and COX III protein content were significantly reduced. The width, length and area of mitochondria in mice treated with flavonoid glycoside were significantly reduced, and ATP level and COX III protein content were significantly increased[35]. At the same time, we detected that the proteins of stress factors GPX4, NOX4, NF2, apoptosis indicators caspase 3, caspase 7, caspase 8 and necrosis indicators TNFRSF1α, RIPK1, RIPK3 in RPE cells of flavonoid glycoside mice and human RPE cells were significantly reduced. These results suggested that flavonoid glycoside can effectively improve the stress and mitochondrial dysfunction of RPE cells, and protect RPE cells by regulating the expression of stress, apoptosis and necrosis indicators[36,37].
In conclusion, flavonoid glycoside can significantly improve the mitochondrial dysfunction and inflammatory oxidative stress of RPE cells and play a good role in protecting macular degeneration.
Funding:
This article is financially supported by Science and Technology Project of Liaoning province (2022JH1/1080071, 2019-BS101), Program project of Liaoning Provincial Department of Education (LJKQZ2021150), Youth fund of the Third Affiliated Hospital of Jinzhou Medical University (FSSYQM2020005).
Conflict of interests:
The authors declared no conflict of interests.
References
- Kaarniranta K, Uusitalo H, Blasiak J, Felszeghy S, Kannan R, Kauppinen A, et al. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog Retinal Eye Res 2020;79:100858.
[Crossref] [Google Scholar] [PubMed]
- Blasiak J. Senescence in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 2020;77(5):789-805.
[Crossref] [Google Scholar] [PubMed]
- Nashine S. Potential therapeutic candidates for age-related macular degeneration (AMD). Cells 2021;10(9):2483.
[Crossref] [Google Scholar] [PubMed]
- Hilely A, Au A, Freund KB, Loewenstein A, Souied EH, Zur D, et al. Non-neovascular age-related macular degeneration with subretinal fluid. Br J Ophthalmol 2021;105(10):1415-20.
[Crossref] [Google Scholar] [PubMed]
- Shu DY, Butcher E, Saint-Geniez M. EMT and EndMT: Emerging roles in age-related macular degeneration. Int J Mol Sci 2020;21(12):4271.
[Crossref] [Google Scholar] [PubMed]
- Ambati M, Apicella I, Wang SB, Narendran S, Leung H, Pereira F, et al. Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc Natl Acad Sci 2021;118(41):e2102975118.
[Crossref] [Google Scholar] [PubMed]
- Sharma R, George A, Nimmagadda M, Ortolan D, Karla BS, Qureshy Z, et al. Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model. Nat Commun 2021;12(1):7293.
[Crossref] [Google Scholar] [PubMed]
- Little K, Llorián-Salvador M, Tang M, Du X, Marry S, Chen M, et al. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J Neuroinflammation 2020;17:1-2.
[Crossref] [Google Scholar] [PubMed]
- Guymer RH, Rosenfeld PJ, Curcio CA, Holz FG, Staurenghi G, Freund KB, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: Classification of atrophy meeting report 4. Ophthalmology 2020;127(3):394-409.
[Crossref] [Google Scholar] [PubMed]
- Manian KV, Galloway CA, Dalvi S, Emanuel AA, Mereness JA, Black W, et al. 3D iPSC modeling of the retinal pigment epithelium-choriocapillaris complex identifies factors involved in the pathology of macular degeneration. Cell Stem Cell 2021;28(5):846-62.
[Crossref] [Google Scholar] [PubMed]
- Orozco LD, Chen HH, Cox C, Katschke KJ, Arceo R, Espiritu C, et al. Integration of eQTL and a single-cell atlas in the human eye identifies causal genes for age-related macular degeneration. Cell Rep 2020;30(4):1246-59.
[Crossref] [Google Scholar] [PubMed]
- Nguyen QD, Das A, Do DV, Dugel PU, Gomes A, Holz FG, et al. Brolucizumab: Evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology 2020;127(7):963-76.
[Crossref] [Google Scholar] [PubMed]
- Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Chen S, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vision Sci Technol 2021;10(10):13.
[Crossref] [Google Scholar] [PubMed]
- Yang M, So KF, Lam WC, Lo AC. Novel programmed cell death as therapeutic targets in age-related macular degeneration? Int J Mol Sci 2020;21(19):7279.
[Crossref] [Google Scholar] [PubMed]
- Zhang M, Jiang N, Chu Y, Postnikova O, Varghese R, Horvath A, et al. Dysregulated metabolic pathways in age-related macular degeneration. Sci Rep 2020;10(1):1-4.
- Wei TT, Zhang MY, Zheng XH, Xie TH, Wang W, Zou J, et al. Interferon-γ induces retinal pigment epithelial cell Ferroptosis by a JAK1-2/STAT1/SLC7A11 signaling pathway in age-related macular degeneration. FEBS J 2022;289(7):1968-83.
[Crossref] [Google Scholar] [PubMed]
- O'Neill HC, Limnios IJ, Barnett NL. Advancing a stem cell therapy for age-related macular degeneration. Curr Stem Cell Res Ther 2020;15(2):89-97.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Garcia J, Usategui-Martin R, Sanabria MR, Fernandez-Perez E, Telleria JJ, Coco-Martin RM. Pathophysiology of age-related macular degeneration: Implications for treatment. Ophthalmic Res 2022;65(6):615-36.
[Crossref] [Google Scholar] [PubMed]
- Jaffe GJ, Chakravarthy U, Freund KB, Guymer RH, Holz FG, Liakopoulos S, et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration: Classification of atrophy meeting report 5. Ophthalmol Retina 2021;5(9):855-67.
[Crossref] [Google Scholar] [PubMed]
- Samra YA, Kira D, Rajpurohit P, Mohamed R, Owen LA, Shakoor A, et al. Implication of N-methyl-d-aspartate receptor in homocysteine-induced age-related macular degeneration. Int J Mol Sci 2021;22(17):9356.
[Crossref] [Google Scholar] [PubMed]
- Khateb S, Jha S, Bharti K, Banin E. Cell-based therapies for age-related macular degeneration. Adv Exp Med Biol 2021;1256:265-93.
[Crossref] [Google Scholar] [PubMed]
- La Cunza N, Tan LX, Thamban T, Germer CJ, Rathnasamy G, Toops KA, et al. Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration. JCI Insight 2021;6(9):e142254.
[Crossref] [Google Scholar] [PubMed]
- Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L, et al. Metformin protects ARPE-19 cells from glyoxal induced oxidative stress. Oxid Med Cell Longev 2020;2020:1740943.
- Tan LX, Germer CJ, La Cunza N, Lakkaraju A. Complement activation, lipid metabolism and mitochondrial injury: Converging pathways in age-related macular degeneration. Redox Biol 2020;37:101781.
[Crossref] [Google Scholar] [PubMed]
- Blasiak J, Szczepanska J, Fila M, Pawlowska E, Kaarniranta K. Potential of telomerase in age-related macular degeneration-involvement of senescence, DNA damage response and autophagy and a key role of PGC-1α. Int J Mol Sci 2021;22(13):7194.
[Crossref] [Google Scholar] [PubMed]
- Pan C, Banerjee K, Lehmann GL, Almeida D, Hajjar KA, Benedicto I, et al. Lipofuscin causes atypical necroptosis through lysosomal membrane permeabilization. Proc Natl Acad Sci 2021;118(47):e2100122118.
[Crossref] [Google Scholar] [PubMed]
- Gupta U, Ghosh S, Wallace CT, Shang P, Xin Y, Nair AP, et al. Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD. Autophagy 2023;19(1):92-111.
[Crossref] [Google Scholar] [PubMed]
- Yang YP, Hsiao YJ, Chang KJ, Foustine S, Ko YL, Tsai YC, et al. Pluripotent stem cells in clinical cell transplantation: Focusing on induced pluripotent stem cell-derived RPE cell therapy in age-related macular degeneration. Int J Mol Sci 2022;23(22):13794.
[Crossref] [Google Scholar] [PubMed]
- Moraes G, Fu DJ, Wilson M, Khalid H, Wagner SK, Korot E, et al. Quantitative analysis of OCT for neovascular age-related macular degeneration using deep learning. Ophthalmology 2021;128(5):693-705.
[Crossref] [Google Scholar] [PubMed]
- Kim SY, Kambhampati SP, Bhutto IA, McLeod DS, Lutty GA, Kannan RM. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp Eye Res 2021;203:108391.
[Crossref] [Google Scholar] [PubMed]
- Flores-Bellver M, Mighty J, Aparicio-Domingo S, Li KV, Shi C, Zhou J, et al. Extracellular vesicles released by human retinal pigment epithelium mediate increased polarised secretion of drusen proteins in response to AMD stressors. J Extracell Vesicles 2021;10(13):e12165.
[Crossref] [Google Scholar] [PubMed]
- Lewis Luján LM, McCarty MF, Di Nicolantonio JJ, Gálvez Ruiz JC, Rosas-Burgos EC, Plascencia-Jatomea M, et al. Nutraceuticals/drugs promoting mitophagy and mitochondrial biogenesis may combat the mitochondrial dysfunction driving progression of dry age-related macular degeneration. Nutrients 2022;14(9):1985.
[Crossref] [Google Scholar] [PubMed]
- Tang Z, Huo M, Ju Y, Dai X, Ni N, Liu Y, et al. Nanoprotection against retinal pigment epithelium degeneration via ferroptosis inhibition. Small Methods 2021;5(12):2100848.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Wang L, Gu S, Yu Y, Huang H, Mo K, et al. D609 protects retinal pigmented epithelium as a potential therapy for age-related macular degeneration. Signal Transduc Target Ther 2020;5(1):20.
[Crossref] [Google Scholar] [PubMed]
- Bharti K, den Hollander AI, Lakkaraju A, Sinha D, Williams D, Finnemann SC, et al. Cell culture models to study retinal pigment epithelium-related pathogenesis in age-related macular degeneration. Exp Eye Res 2022;222:109170.
[Crossref] [Google Scholar] [PubMed]
- Ebeling MC, Geng Z, Stahl MR, Kapphahn RJ, Roehrich H, Montezuma SR, et al. Testing mitochondrial-targeted drugs in iPSC-RPE from patients with age-related macular degeneration. Pharmaceuticals 2022;15(1):62.
[Crossref] [Google Scholar] [PubMed]
- Wu DM, Ji X, Ivanchenko MV, Chung M, Piper M, Rana P, et al. Nrf2 overexpression rescues the RPE in mouse models of retinitis pigmentosa. JCI Insight 2021;6(2):e145029.
[Crossref] [Google Scholar] [PubMed]