- *Corresponding Author:
- J. L. Wang
School of Public Health, the Key laboratory of Environmental Pollution Monitoring and Diseases Control, Ministry of Education, Guizhou Medical University, Guiyang 550025, Guizhou, China
E-mail: wjlrjg@126.com
| This article was originally published in a special issue, “Clinical Research in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2021:83(1)spl issue “162-168” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Pepper wilt disease is common in the production of pepper in Guizhou province, China and fenaminosulf is often used to prevent its occurrences in agricultural practices. To estimate the effects of fenaminosulf on the sustainable pepper production, a pot experiment for 93 d was conducted to investigate the effects of fenaminosulf on the growth of pepper (Capsicum annuum L.), microbial communities and enzyme activities of the soil infested with phytopathogen Fusarium oxysporum. Four treatments: T1-seeding a week after pathogen inoculation in the soil, T2-seeding and soil drenching of fenaminosulf (at recommended dose) a week after pathogen inoculation in the soil, T3-pathogen inoculation in the soil at the first four to fiveleaf stage of pepper seedling and T4-soil drenching of fenaminosulf (at recommended dose) a week after pathogen inoculation in the soil at the first four to five-leaf stage of pepper seedling were established. The studies showed that T2 and T4 of fenaminosulf application obviously improved pepper survival rate, partially inhibited plant growth and development and significantly reduced soil bacterial quantity, but had no similar effects on the soil activities of catalase, urease and alkaline phosphatase. On the basis of stronger growth potential of plant, larger quantities of bacteria and actinomycetes and higher activities of catalase and alkaline phosphatase in the soil, T4 strategy might be reasonable for the production of pepper in the field where pepper wilt disease usually occurs.
Keywords
Fenaminosulf, pepper, soil microbial community, soil enzymatic activity
Pepper (Capsicum spp.) is one of the important vegetables in Guizhou province of China, which at least has fifteen local varieties. Pepper wilt disease is a commonly severe root disease causing great losses in pepper production of many planting regions and finally results in reduced agriculture income. In the plantation of Guizhou province, Pepper wilt disease is mainly caused by Fusarium oxysporum, a soilborne pathogen, which incurs the sequential appearance of different symptoms on hosts including first vein clearing on the younger leaves and then epinasty on the older ones and further withering and defoliation of old leaves and plant stunting and finally the death of plants. In the conventional cropping system, the control of pepper wilt disease is achieved through the use of fungicides besides carbendazim, topsin-M, mancozeb, kocide, etc. However some specific fungicides are getting less effective over time because of the pathogen?s acquired resistance to fungicide. Therefore it is inevitable to resort to the alternatives of other fungicides to control the occurrence of Fusarium wilt disease, and fenaminosulf is the popular one of other fungicides selected for control of Fusarium wilt disease in Guizhou province of China mainly in the mode of soil drenching. As a fungicide, fenaminosulf is registered for uses on ornamental plant, sugarcane, avocado, lawn and turf[1] and also permitted for the fungicidal treatments of some seeds, which included the bean, beet, corn, cotton, cucumber, sorghum and spinach, etc.[1]. Hitherto, there are few reports about effects of fenaminosulf on the growth of pepper infected with Fusarium oxysporum. With this idea the present investigation has been set to study the effects of fenaminosulf on the growth of pepper infected with Fusarium oxysporum. Meanwhile, the effects of fenaminosulf on microbial communities and enzyme activities of the soil were also studied to assess the soil fertility for pepper production of continuous cropping systems.
Materials and Methods
The pot experiments were conducted in the intelligent greenhouse at Guizhou University between July 7, 2015 to October 5, 2015.
Determination of soil characteristics
Fresh soils were gathered in Guizhou University (China) and sieved (2 mm) to remove detritus. The physical and chemical properties of the soils collected were determined by conventional laboratory methods popularly utilized in chemical soil laboratories (Table 1).
| Parameter | Sampled soils |
|---|---|
| pH value | 6.7±0.8 |
| Total salt content (g kg-1) | 0.7±0.03 |
| Organic matter (%) | 17.1±1.3 |
| Alkaline solved N (mg kg?1) | 103.6±2.6 |
| Available P (mg kg-1) | 85.4±1.6 |
| Available K (mg kg-1) | 129.1±2.7 |
Table 1: Chemical Properties of the Soils used in this Study
Experimental design
The soil sample collected was uniformly mixed, and then subdivided into four portions of different treatments for the pot experiments: seeding a week after pathogen inoculation in the soil (T1), seeding and soil drenching of fenaminosulf at recommended dose (0.05 %) (50 % wettable power, Dandong Agrochemical Plant, Liaoning Province, China) a week after pathogen inoculation in the soil (T2), pathogen inoculation in the soil at the first four to five-leaf stage of pepper seedling (T3) and soil drenching of fenaminosulf (0.05 %) a week after pathogen inoculation in the soil at the first four to five-leaf stage of pepper seedling (T4). The preparation of pathogen inoculums, Fusarium oxysporum was done as followings: the purified phytopathogenic strain was activated on potato dextrose agar medium (PDA) (2 % potato, 2 % dextrose and 1.8 % agar) slant and then transferred to twenty PDA plates for incubation at 28° for 5 d. A large quantity of mycelia was gathered by scraping the colonies on PDA plates with the stainless blades and then immersed in the sterile water for stirring 30 min. The suspensions of mycelia were filtered with three layers of cotton cloth and the final filtrates, viz. spore suspensions (1010 spores/mL) were achieved for the inoculums of the soil in pots. The soil in each pot was sprayed 200 mL spore suspensions for imitation of the soil infested with Fusarium oxysporum. 0.05 % fenaminosulf solution was applied as soil drenching at 150 mL each pot to get 60 % of the maximum water holding capacity for the moisture content of the soil. Pathogen inoculation and soil drenching with fenaminosulf were done once for all the treatments. The pot experiments were conducted in a glasshouse where day/night temperature was approximately 26/19° up to 56 d and 21/16° from d 56 to d 93 after seeding. The incubation period of 93 d fully potentiated the development and growth of pepper for all the treatments. Each treatment was performed in triplicate, which required forty five pepper seeds (100 % germination rate) in total (15 seeds per pot). Water was regularly sprayed to ensure the wetness of the soil. After the incubation period finished, survival rate of plants was investigated and also height of plants was measured immediately, while the dry weight of stem and root for plant was weighed respectively until constant weight was reached in a ventilated heater at 60° on each treatment.
Soil microbial community analysis
Most probable number method (MPN)[2] was used to estimate the quantity of soil microbial population, viz. heterotrophic bacteria, actinomycetes and fungi). The fresh soil samples (3 g per sample) were taken from the soils of three pots for each treatment, homogenized in 10 ml of 0.85 % (wt/vol) saline, shaken for 20 min at 250 rpm and stationed for 5 min. The suspension was diluted serially in sterile glass tubes containing 9 mL of sterile water. Nutrient broth medium(1.0 % peptone, 0.3 % beef extract, 0.5 % sodium chloride (NaCl), pH 7.2 ), Gauze’s Medium NO.1 (2 % soluble starch, 0.1 % KNO3, 0.05 % NaCl, 0.05 % MgSO4•7H2O, 0.05 % K2HPO4, 1.0 % MgSO4•7H2O, pH 7.4) and Martin medium (0.5 % peptone, 1.0 % dextrose, 0.1 % KH2PO4, 0.5 % MgSO4•7H2O, 0.003 % Rose Bengal, 0.003 % streptomycin added after autoclaving) were employed for the enrichment culture of heterotrophic bacteria, actinomycetes and fungi, respectively. The test tube was incubated at 28° for 36 h, 5 d and 2 d for nutrient broth medium, Gauze’s Medium No.1 and Martin medium. The number of tubes showing microbial growth and multiplication is scored and then the quantity of microbial population in per gram of soil sample was calculated on the basis of the microbial quantity achieved from the MPN table and gram numbers of soil sample. Each treatment was replicated three times for counting of microbial population.
Soil enzyme assays
Activities of soil enzymes including catalase, urease and alkaline phosphatase were assayed in triplicate air-dried samples. Catalase activity was determined by back-titrating residual hydrogen peroxide (H2O2) with KMnO4 and was expressed as ml H2O2-consumption g-1 soil[3]. Urease activity was assayed using the method described by May and Douglas[4], expressed as mg ammonia nitrogen 100 g-1 soil. Alkaline phosphatase activity was measured using disodium phenyl phosphate colorimetric method[5], expressed as mg phenol 100 g-1 soil.
Statistical analysis
Analysis of variance using one-way ANOVA was carried out, and all significance analyses were performed by Duncan’s multiple-range test at a significance level of p=0.05 using SAS 8.1 for Windows 7.
Results and Discussion
The application of fungicides in agricultural production is unavoidable to satiate the food requirements of the increasing population of human. As a fungicide, fenaminosulf is suited only for soil and seed application, due to its instability to light[6]. Therefore the method of soil drenching of fenaminosulf was adopted in the present study. Obtained results of Mani et al.[7] indicated that planting date and fungicide timing had the important effects on plant disease protection and crop yield. Therefore two different strategies of fungicide application were evaluated in present study to achieve a better management of pepper production. Compared with T1, T2 improved the control efficacy of Fusarium wilt disease of pepper, but significantly reduced plant height and biomass (stem and root dry weights) (Table 2). On another treatment pair (T3 and T4), T4 also significantly enhanced plant protection against pepper Fusarium wilt disease and reduced the plant biomass, but had no significant effect on plant height as compared with T3.
| Treatment | Survival rate (%) |
Plant height (cm) |
Stem dry weight (g plant-1) |
Root dry weight (g plant-1) |
|---|---|---|---|---|
| T1 | 50.6±1.7b | 20.2±1.2a | 0.09±0.001c | 0.04±0.002c |
| T2 | 86.7±2.3a | 14.0±1.0b | 0.04±0.001d | 0.02±0.001d |
| T3 | 86.7±2.3a | 24.1±1.3a | 0.23±0.003a | 0.10±0.004a |
| T4 | 88.9±2.0a | 25.0±1.2a | 0.18±0.002b | 0.07±0.002b |
Table 2: Effect of Soil Drenched with Fenaminosulf on Survival Rate and Growth of Pepper Plant Inoculated with Fusarium Oxysporum
Whatever on plant biomass or plant height, T2 had the stronger inhibitory effects on plant than T4; however on survival rate of plant, T2 displayed the similar control efficacy of pepper wilt disease caused by Fusarium oxysporum as T4. From the above mentioned, T4 was more practical than T2 for the production of pepper infected with Fusarium oxysporum in the fields, which attribute to the close correlation between plant size and yield under plant disease stress[8]. In terms of the protective effects of pepper, application date of fenaminosulf did not affect its control efficacy of pepper wilt disease. However the present results that soil application of fenaminosulf for the control of pepper wilt disease obviously reduced plant biomass and height of pepper, which corresponded with the previous studies of yield loss and plant stunting on the controls of sugar-beet blackleg disease caused by Aphanomyces cochlioides[9] and maize seedling disease by Aspergillus sp., Diplodia sp., Fusarium sp. and Pythium sp.[10] with soil drenching of fenaminosulf, respectively. The relative inhibitory effects of T2 and T4 on plant height, stem dry weight and root dry weight were 30.7, 55.6, 50.0 % and 3.7, 21.7, 30 % in comparison with T1 and T3, respectively, indicating that the application date of fenaminosulf had the significant effects on plant growth and development, which was evidenced by Hausbeck et al.[11] on Geraniums with fenaminosulf for root and stem rot disease control. In addition, the study on the inhibition of Sunn hemp (Crotalaria juncea) by dexon (p-dimethylaminobenzenediazo sodium sulfonate) revealed that this efficacy was closely related to the inhibitory effect of dexon on plant metabolism particularly hormone rather than on rhizobia[12]. In particular, the activity of root metabolism affects the physicochemical conditions of the rhizosphere surroundings which consequentially influence the microbial population of rhizosphere. Changes in soil physicochemical conditions can determine the availability and cycling of nutrients mediated by the activities of soil microbes including elements/compounds which may be toxic for plants and microorganisms[13].In the present study, fenaminosulf treatments had the detrimental effects on plant growth and development, which might be explained by the restriction of the various microbial populations and their activities[14].
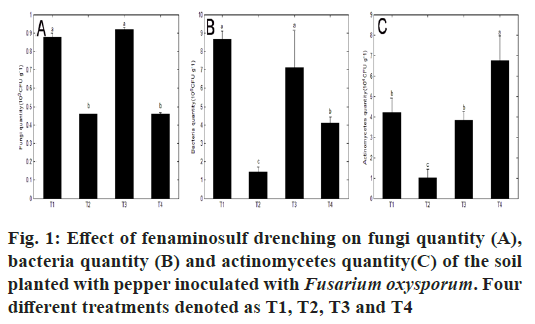
Various pesticides applied for plant disease control ultimately drop into the soil and may have positive or negative effects on plant growth owing to their influence on the composition and activity of soil microorganism. The composition of microbial communities in the soil is the important indicators of soil quality. These microbial communities are responsible not only for maintaining soil fertility[15] but also for removing soil contaminants like pesticides from soil[16]. With the extensive application of pesticides in agricultural production, it is not neglectful for the effects of pesticides on soil microbial community structure. Obtained results of previous studies indicated the effects of pesticides on soil microbial community were correlated with multifarious factors including pesticide use/type/ dosage[17], type of soil, time of incubation[18] or the management of the soils[19]. In the present study, effects of fenaminosulf on the composition of soil microbial community were elaborated in details (fig. 1). No differences existed on the numbers of fungi (fig. 1A), bacteria (fig.1B) and actinomycetes (fig. 1C) between T1 and T3, which indicated the non-interruption of Fusarium oxysporum from the natural soil environment on the soil microbial communities. Compared with T1, T2 (soil application of fenaminosulf) significantly reduced the population of fungi (fig. 1A), bacteria (fig. 1B) and actinomycetes (fig. 1C) in the soil, especially the quantity of bacteria was reduced approximately by 83.3 % (fig. 1B), which was similar to the results reported by Zhang et al.[20] of the impacts of fungicide fluopyram tested at all doses on the bacteria and fungi in the silty-loam agricultural soil and Mahapatra et al.[21] of the effects of imidacloprid application at four different doses on bacteria, actinomycetes and fungi in rice soil. The measured microbial community shifts might be related to the mortality of fenaminosulfdegrading metabolites. However in comparison with T3, T4 (soil application of fenaminosulf) significantly reduced the population of fungi (fig. 1A) and bacteria (fig. 1B) by 50 % and 42.2 %, respectively, but strikingly increased the population of actinomycetes (fig. 1C), which were of great importance in the decomposition of soil organic matter and the liberation of its nutrients, especially for cellulose, chitin and phosphlipids. Obtained results of the previous study by Ba?maga et al.[22] also indicated that soil contamination with higher dose of 80-fold herbicides Lumax 537.5SE contributed to elevated counts of actinomycetes. The increase in the quantity of actinomycetes might be related to the rapid propagation of specific fenaminosulf-degrading species induced by the higher levels of fenaminosulf residue owing to its shorter incubation time in the soil or the present soil properties of applied fungicide, e.g., pH, which was negatively correlated with the abundance of actinomycetes in the soil[23]. On the whole, there was a decreasing tendency on the total quantity of fungi, bacteria and actinomycetes tested in comparison with their respective controls, whatever for T2 or T4, which was contrary to the previous report for the stimulating effect of dexon (p-dimethylaminobenzenediazo sodium sulfonate) on the total quantity of fungi, bacteria and actinomycetes[24], but conformed to the result of the soil application of metalaxyl[25] and herbicide[26] resulting in a significant reduction in micrbial biomass.
Although the same tendencies of the reducing population of fungi and bacteria existed for T2 and T4, a sharp increase of the quantity of actinomycetes only appeared in T4, which implied that incubation time of fenaminosulf was related to the response of microbial communities to applied fungicide. Similarly, some other studies had found that incubation time was a significant factor affecting the soil microbial community structure in response to antibiotics[27]. In this study, the effects of T2 and T4 on the constituent of soil microbial communities were not totally identical, when fenaminosulf drenched at plant different developmental stage, which might be elucidated with the viewpoint of Pei et al.[28]. Soil microbial community abundance and structure could be significantly influenced by both plant functional traits and local soil characteristics, especially for soil chemical characteristics[29]. T4 significantly increased the quantities of soil bacteria and actinomycetes as collated with T2, which might further promote the service function of bacterial communities in the soil in the ecosystem[30] and finally led to better growth and development of plant facilitated by soil bacteria community involved in microbial mineralization of organic compounds and associated biotransformations such as nutrient dynamics and their bioavailability. Therefore as viewed from soil microbial communities, it is preferred to implement T4 schedule for pepper production in the fields, where pepper wilt disease frequently occurs.
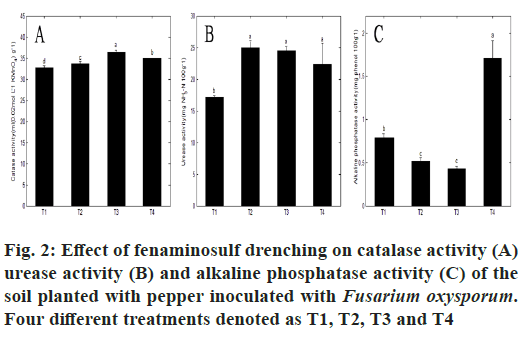
As one of pesticides in agriculture, fungicide can guarantee the higher production of crops, but its deleterious effects on natural environment, especially on the soil environment through disturbing its homeostasis are not ignored. Soil enzymes are secreted by many organisms, but most often originate from soil microorganisms. The activities of soil enzymes influence many soil biological processes related with soil fertility. They are intimately involved in the cycling of nutrients, affect the efficiency of fertilizers, reflect the microbial activity in the soil and act as indicators of soil change[31]. Catalase in soil has been related to both the number of aerobic microorganisms and soil fertility in the soil[32] and is also often used to indicate the microbial antioxidation ability[33], which is stimulated when slightly toxic compounds are present, and inhibited when the toxicity increases[34]. Urease plays a very important role in the nitrogen cycle in soils, and is strongly correlated to soil organic matter (SOM) content[35] and microbial biomass[36]. Phosphatase enzymes (acid and alkaline phosphatase) are a good indicator of the organic phosphorous mineralization potential and biological activity of soils[37]. Therefore in the present study, the activities of three enzymes viz. catalase, urease and alkaline phosphatase were determined to study the effect of fenaminosulf on enzymatic activities of planting soil infested with Fusarium oxysporum (fig. 2). Two standard curves, y=0.3716x−0.0174 (R=0.9947) and y=0.038x−0.0018 (R=0.9953) were made for the result’s calculation of the urease and alkaline phosphatase activities, respectively. Although the inoculation of Fusarium oxysporum did not cause the changes on soil microbial communities for T1 and T3 (fig. 1A, fig. 1B and fig. 1C), the obvious differences existed on the soil enzymatic activities, which indicated that the shifts of soil physiochemical property might be associated with the difference of plant traits. In terms of treatment pair of T1 and T2, T2 (soil application of fenaminosulf) increased the activities of catalase and urease by 3.07 % and 40.60 %, respectively, but reduced alkaline phosphatase by 34.21 %, which indicated the inhibitory effects of fenaminosulf used on alkaline phosphatase and the stimulus on catalase and urease at the end of the fenaminosulf incubation of 93 d. On another treatment pair of T3 and T4, T4 (soil application of fenaminosulf) significantly increased the activity of alkaline phosphatase by 297.67 %, while reduced the activities of catalase and urease by 3.67 % and 9.08 % which indicated the inhibitory effects of fenaminosulf used on soil catalase and urease and the stimulus on soil alkaline phosphatase at the end of the fenaminosulf incubation of 70 d. The above mentioned results demonstrated that the effects of fenaminosulf on the activities of catalase, urease and alkaline phosphatase were associated with the incubation time of fenaminosulf in the soil.
The alteration of incubation time led to the changes in the amounts of soil fenaminosulf residues due to microbial transformation[38] or photochemical decomposition[6], which still affected the soil biochemical processes catalyzed by enzymes. In the present study, the variances on catalase, urease and alkaline phosphatase for T2 and T4 might be ascribed to the differences on the relative amounts of fenaminosulf residues in the soil for the different incubation time. Due to the instability of fenaminosulf in the soil, the relative amount of soil fenaminosulf residues for T4 in the shorter incubation time was superior to that for T2. The differences on the activity of catalase between two treatment pairs (T1 and T2, T3 and T4) were obviously affected by the relative amount of soil fenaminosulf residues for different incubation time, viz. stimulation when lower dose (incubation time of 93 d for T2) and inhibition when higher dose (incubation time of 70 d for T4) in comparison with respective control, which coincided with the viewpoint of Shiyin et al.[34] on effects of pesticide on catalase activity. The variable results on soil alkaline phosphatase between two treatment pairs also revealed that the incubation time significantly affected the alkaline phosphatase activity, which increased in the shorter incubation time, but decreased in the longer time as compared with respective controls. Similar results were reported by Rasool and Reshi[39] on the fluctuation of alkaline phosphatase varying with the incubation time by normal application rate of 60 kg ha-1 of mancozeb in soil.
This variable results of two different treatment pairs on alkaline phosphatase might be explained by primarily different mechanisms that pesticide degradation products were responsible for inhibition to alkaline phosphatise (T2 as collated with T1) or the shift in the composition of the soil microbial community contributed to the increment in the activity of alkaline phosphatase related to phosphorous bio-availability (T4 as collated with T3)[40]. The stability of urease between two different treatment pairs might imply that this enzyme had not any role in the degradation pathway of the fungicides or no specific substrates in the currently contaminated soil ecosystems, which was similar to the results of Moreno et al.[41] on the activities of soil urease affected by different concentration atrazine with different incubation time and Du et al.[42] on the same enzyme activities affluenced by mesotrione of different doses at different time interval, but also there existed some cases on the variable activities of urease influenced by pesticides with different concentrations at different time points[43,44]. The higher activity of catalase for T4 attributed not only to the activity of soil microbial microflora, but also contribution of plant root stressed by pathogen Fusarium oxysporum, which help plant overcome the detrimental environment for surviving. The higher activity of alkaline phosphatase for T4 principally derived from soil microbial population, not from plants[45] easily facilitated the mineralization of organic matter[37], which was available to plant growth and development. Therefore as viewed from soil enzymes, the scheme of T4 was suitable for the soil application of fenaminosulf for management of pepper in the field.
Fenaminosulf at recommended dose obviously decreased plant biomass of pepper, but significantly enhanced plant protection against pepper Fusarium wilt disease; Fenaminosulf at recommended dose might alter the microbial population balance in the soil, as evidenced by the changes of soil microbial quantity and enzymatic activity; T4 strategy was more reliable than T2 for the wilt disease management of pepper in the field.
Acknowledgements
The authors acknowledge the National Natural Science Foundation of China, China (31760604, 31960265) and the First-Class Discipline Construction Project in Guizhou Province-Public Health and Preventive Medicine (NO.2017 [85]) for their financial support.
Conflict of interest
The authors declare that they have no competing interests.
References
- Liman R, Ci?erci ?H, Aky?l D, Eren Y, Konuk M. Determination of genotoxicity of Fenaminosulf by Allium and Comet tests. Pestic Biochem Phys 2011;99(1):61-4.
- Cai Z, Li S, Zhang W, Ma J, Wang J, Cai J, et al. Effects of the novel pyrimidynyloxybenzoic herbicide ZJ0273 on enzyme activities, microorganisms and its degradation in Chinese soils. Environ Sci Pollut Res 2015;22(6):4425-33.
- Lu L, Huggins T, Jin S, Zuo Y, Ren ZJ. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ Sci Technol 2014;48(7):4021-9.
- May PB, Douglas LA. Assay for soil urease activity. Plant Soil 1976;45(1):301-5.
- Zhou LK. Soil Enzymology. Beijing: Science Press; 1987.
- Hills FJ, Leach LD. Photochemical decomposition and biological activity of p-dimethylaminobenzenediazo sodium sulfonate (Dexon). Phytopathology 1962;52(1):51-6.
- Mani KK, Hollier CA, Groth DE. Effect of planting date, fungicide timing and cultivar susceptibility on severity of narrow brown leaf spot and yield of rice. Crop Prot 2016;90:186-90.
- MONTGOMERIE IG, KENNEDY DM. The effects of dazomet, fenaminosulf, and soil ridges on red core disease of strawberry. Ann Appl Biol 1982;100(3):443-55.
- Byford WJ, Prince J. Experiments with fungicides to control Aphanomyces cochlioides in sugar beet. Ann Appl Biol 1976; 83: 69-77. Byford WJ, Prince J. Experiments with fungicides to control Aphanomyces cochlioides in sugar beet. Ann Appl Biol 1976;83(1):69-77.
- Falloon RE. Fungicide seed treatment of maize to improve establishment and control seedling pathogens. New Zeal J Exp Agr 1982;10(2):197-202.
- Hausbeck MK, Stephens CT, Heins RD. Size and flowering of seed-propagated geraniums in response to fungicide drenching schedules. HortScience 1990;25(6):644-6.
- Karanth NG, Vasantharajan VN. Phytotoxicity of dexon (p-dimethylaminobenzenediazo sodium sulfonate) towards the legume Crotalaria juncea. Proc Natl Acad Sci India B 1975;40:576-85.
- Neumann G, George TS, Plassard C. Strategies and methods for studying the rhizosphere-the plant science toolbox. Plant Soil 2009;321(1):431-56.
- El-Abyad MS, Ghareeb M. Changes of tomato rhizosphere microflora following application of the herbicide diphenamid to soil infested with Fusarium oxyspomm f. sp. lycopersici. Mycopathologia 1991;113(2):89-94.
- Schneider T, Keiblinger KM, Schmid E, Sterflinger-Gleixner K, Ellersdorfer G, Roschitzki B, et al. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J 2012;6(9):1749-62.
- Aislabie J, Lloyd-Jones G. A review of bacterial-degradation of pesticides. Soil Research 1995;33(6):925-42.
- Xu J, Zhang Y, Dong F, Liu X, Wu X, Zheng Y. Effects of repeated applications of chlorimuron-ethyl on the soil microbial biomass, activity and microbial community in the greenhouse. B Environ Contam Tox 2014;92(2):175-82.
- WU XH, Jun XU, LIU YZ, DONG FS, LIU XG, ZHANG WW, et al. Impact of fluxapyroxad on the microbial community structure and functional diversity in the silty-loam soil. J Integr Agr 2015;14(1):114-24.
- Su?owicz S, Cyco? M, Piotrowska-Seget Z. Non-target impact of fungicide tetraconazole on microbial communities in soils with different agricultural management. Ecotoxicology 2016;25(6):1047-60.
- Zhang Y, Xu J, Dong F, Liu X, Wu X, Zheng Y. Response of microbial community to a new fungicide fluopyram in the silty-loam agricultural soil. Ecotox Environ Safe 2014;108:273-80.
- Mahapatra B, Adak T, Patil NK, Gowda GB, Jambhulkar NN, Yadav MK, et al. Imidacloprid application changes microbial dynamics and enzymes in rice soil. Ecotox Environ Safe 2017;144:123-30.
- Ba?maga M, Kucharski J, Wyszkowska J, Tomkiel M, Borowik A. Response of actinomycetes, phosphatases and urease to soil contamination with herbicides. Ecol Chem Eng S 2015;22(2):255-67.
- Li R, Khafipour E, Krause DO, Entz MH, de Kievit TR, Fernando WD. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PloS one 2012;7(12):e51897.
- Karanth NK, Chitra C, Vasanthrajan VN. Behavior of the fungicide Dexon in soil. J Indian I Sci 1974;56(6):290.
- Sukul P. Enzymatic activities and microbial biomass in soil as influenced by metalaxyl residues. Soil Biol Biochem 2006;38(2):320-6.
- Tomkiel M, Ba?maga M, Borowik A, Kucharski J, Wyszkowska J. Effect of a mixture of flufenacet and isoxaflutole on population numbers of soil-dwelling microorganisms, enzymatic activity of soil, and maize yield. J Environ Sci Heal B 2019;54(10):832-42.
- Cui H, Wang SP, Fu J, Zhou ZQ, Zhang N, Guo L. Influence of ciprofloxacin on microbial community structure and function in soils. Biol Fertil Soils 2014;50(6):939-47.
- Pei Z, Eichenberg D, Bruelheide H, Kröber W, Kühn P, Li Y, et al. Soil and tree species traits both shape soil microbial communities during early growth of Chinese subtropical forests. Soil Biol Biochem 2016;96:180-90.
- Massenssini AM, Bonduki VH, Melo CA, Tótola MR, Ferreira FA, Costa MD. Relative importance of soil physico-chemical characteristics and plant species identity to the determination of soil microbial community structure. Appl Soil Ecol 2015;91:8-15.
- Strickland MS, Lauber C, Fierer N, Bradford MA. Testing the functional significance of microbial community composition. Ecology 2009;90(2):441-51.
- Dick WA, Cheng L, Wang P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 2000;32(13):1915-9.
- Trasar-Cepeda C, Camiña F, Leirós MC, Gil-Sotres F. An improved method to measure catalase activity in soils. Soil Biol Biochem 1999;31(3):483-5.
- Caldwell BA. Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 2005;49(6):637-44.
- Shiyin L, Lixiao N, Panying P, Cheng S, Liansheng W. Effects of pesticides and their hydrolysates on catalase activity in soil. Bull Environ Contam Toxicol 2004;72(3):600-6.
- Myers MG, McGarity JW. The urease activity in profiles of five great soil groups from northern New South Wales. Plant Soil 1968;28(1):25-37.
- Nannipieri P, Pedrazzini F, Arcara PG, Piovanelli C. Changes in amino acids, enzyme activities and biomasses during soil microbial growth. Soil Sci 1979;127(1):26-34.
- Speir TW, Ross DJ. Soil phosphatase and sulphatase. Soil enzymes 1978;203:197-250.
- Karanth NG, Bhat SG, Vaidyanathan CS, Vasantharajan VN. Conversion of Dexon (p-dimethylaminobenzenediazo sodium sulfonate) to N, N-dimethyl-p-phenylenediamine by Pseudomonas fragi Bk9. Appl Microbiol 1974;27(1):43-6.
- Rasool N, Reshi ZA. Effect of the fungicide Mancozeb at different application rates on enzyme activities in a silt loam soil of the Kashmir Himalaya, India. Trop Ecol 2010;51(2):199.
- DeForest JL, Scott LG. Available organic soil phosphorus has an important influence on microbial community composition. Soil Sci Soc Am J 2010;74(6):2059-66.
- Moreno JL, Aliaga A, Navarro S, Hernández T, García C. Effects of atrazine on microbial activity in semiarid soil. Appl Soil Ecol 2007;35(1):120-7.
- Du Z, Zhu Y, Zhu L, Zhang J, Li B, Wang J, et al. Effects of the herbicide mesotrione on soil enzyme activity and microbial communities. Ecotox Environ Safe 2018;164:571-8.
- Wang F, Li X, Zhu L, Du Z, Zhang C, Wang J, et al. Responses of Soil Microorganisms and Enzymatic Activities to Azoxystrobin in Cambisol. Pol J Environ Stud 2018;27(6).
- Zhang C, Zhou T, Zhu L, Du Z, Li B, Wang J, et al. Using enzyme activities and soil microbial diversity to understand the effects of fluoxastrobin on microorganisms in fluvo-aquic soil. Sci Total Environ 2019;666:89-93.
- Dick WA, Tabatabai M. Kinetic parameters of phosphatases in soils and organic waste materials. Soil Sci 1984;137(1):7-15.