- *Corresponding Author:
- Zhen Xie
Department of Gynecology, Xingtai People's Hospital, Xingtai, Hebei Province 054001, China
E-mail: 2005rmyy@163.com
| Date of Received | 25 February 2023 |
| Date of Revision | 07 October 2023 |
| Date of Accepted | 12 June 2024 |
| Indian J Pharm Sci 2024;86(3):1076-1082 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of bevacizumab on epithelial ovarian cancer by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor and phosphoinositide 3-kinase/protein kinase B pathway. OVCAR3 cells were divided into control (group A) and experimental (group B) group. The group A did not receive any treatment, while the group B treated human ovarian cancer cells OVCAR3 with bevacizumab. The cell proliferation was detected by cell counting kit 8, the cell migration and invasion were detected by Transwell assay, and the apoptosis was detected by flow cytometry. The expression of proteins was detected by Western blot. The proliferation, migration and invasion of ovarian cancer cells in the group B were decreased. The apoptosis rate of ovarian cancer cells in the group B was increased, the phosphorylated-phosphoinositide 3-kinase and phosphorylated-protein kinase B protein in the group B was reduced than group A. The vascular endothelial growth factor in ovarian cancer cells in the group B was down-regulated and the vascular endothelial growth factor receptor was increased. Bevacizumab inhibits the development and metastasis of ovarian cancer by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor and phosphoinositide 3-kinase/protein kinase B pathways, which may be a potential strategy for the treatment of the disease.
Keywords
Bevacizumab, vascular endothelial growth factor, phosphoinositide 3-kinase/protein kinase B, ovarian cancer
Epithelial Ovarian Cancer (OV) is the common malignant tumors in female reproductive system, and its incidence ranks 3rd in gynecological reproductive system tumors. The occurrence of the disease is a complex process with multi-step and multi-factor participation[1]. At present, the main method to treat OV is surgical resection of tumor cells and systemic chemotherapy supplemented with platinum drugs and paclitaxel[2]. Because of its deep anatomical location, hidden onset and atypical early symptoms, OV is often diagnosed in the middle and late stage, and the prognosis of patients is not ideal. It is reported that invasion and metastasis are the main causes of clinical treatment failure and death in patients with OV, so inhibiting the invasion and metastasis of OV cells is very important for the treatment of OV[3]. Vascular endothelial growth factor (VEGF) is a highly specific molecule that regulates the dynamic balance between angiogenesis and angiogenesis. Together with VEGFR, it participates in the regulation of tumor cell proliferation, differentiation and apoptosis, and affects the progression of OV and other malignant tumors[4].Studies have shown that Bevacizumab exerts its anti-tumor effect by blocking the binding of VEGF to its receptor and by inhibiting tumor angiogenesis[5]. Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT) pathway has been confirmed to be phosphorylated and activated in a variety of human cancer tissues, participates in the process of anti-apoptosis and regulates tumor angiogenesis. Inhibition of PI3K/AKT signal pathway phosphorylation can inhibit tumor angiogenesis[6]. It is not clear whether bevacizumab affects the progression of OV by regulating VEGF/VEGFR and PI3K/AKT pathways. Therefore, this study investigated the effect of bevacizumab on VEGF expression and angiogenesis mediated by Reduced Expression in Immortalized Cells (REIC)/Dickkopf (Dkk)-3 in OV.
Materials and Methods
Cell culture:
Human OV cell line OVCAR3 cells were purchased from the cell bank of Chinese Academy of Sciences and cultured in 10 % Fetal Bovine Serum (FBS) medium at 37° and 5 % Carbon dioxide (CO2) concentration. This experiment was approved by the ethics committee of our hospital.
Experimental reagents and instruments:
FBS and Dulbecco’s Modified Eagle Medium (DMEM) medium were purchased from American Gibco company, 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) from Shanghai Shenggong Bioengineering Co., Ltd., apoptosis detection kit from Beijing Qunxiao Keyuan Biotechnology Co., Ltd., rabbit antihuman uncut caspase-3 (pro-caspase-3) and activated caspase-3 (cleaved caspase-3) antibodies were purchased from SantaCruz company. Inverted fluorescence microscope was purchased from Nanjing Beidan Medical Co., Ltd.; enzyme labeling instrument was purchased from Shandong Jingdao Optoelectronic Technology Co., Ltd.; AnnexinV-Fluorescein Isothiocyanate (FITC)/ Propidium Iodide (PI) apoptosis kit was purchased from Wuhan Punosai Life Technology Co., Ltd.) and Electrochemiluminescence (ECL) luminous reagent was purchased from Hunan Yinuoweizhen Technology Co., Ltd.
Drug treatment:
Human OV cells OVCAR3 were selected as the object of study and were divided into two groups; control group (group A) and experimental group (group B). The group A did not receive any treatment, while the group B used bevacizumab (Shanghai Roche Pharmaceutical Co., Ltd., Batch No: S20210044) 50 μg/ml to treat OV cells OVCAR3.
Cell viability detection:
The cell viability was detected by Cell Counting Kit 8 (CCK8) counting kit, and the two groups of human OV cells OVCAR3 were treated respectively. 5×103 cells were inoculated in each hole. CCK8 solution was added after 48 h of culture, and then the absorbance of cells in each group was detected by enzyme labeling instrument.
Detection of cell migration and invasion ability:
The ability of cell migration and invasion was detected by Transwell method. Human OV OVCAR3 cells in logarithmic growth phase were put into the superior chamber of Transwell, which could be coated or not coated with Matrigel matrix glue. The culture medium containing FBS was added to the lower chamber of Transwell. After 12 h of culture, the superior ventricular cells were removed; the inferior ventricular cells were fixed and stained, and observed by fluorescence microscope.
Apoptosis detection:
Annexin V-FITC double staining was used to detect the apoptosis of human OV cells OVCAR3. The two groups of cells were inoculated in a 6-well plate. After 24 h of culture, the cells were washed with PBS and then re-suspended in binding buffer, Annexin V-FITC reagent and PI staining solution were added. After incubation, the level of apoptosis was detected by kit instructions.
Western blot method:
The expressions of VEGF/VEGFR and PI3K/ AKT pathway proteins were detected by Western blot method. Two groups of treated OVCAR3 cells were used to extract protein and detect the protein content. The proteins were separated by electrophoresis, transferred into membranes, then sealed, incubated overnight at room temperature with antibodies against VEGF/VEGFR and PI3K/ AKT pathway proteins and monoclonal antibodies against β-actin (Abcam company, dilution ratio 1Rom 2000), incubated with secondary antibodies (Abcam company, dilution ratio 1100 000) at room temperature for 1 h, developed with ECL luminescent reagent, measured photosensitive bands with ImageJ software, and used β-actin as internal reference. The relative expression levels of VEGF/VEGFR and PI3K/AKT pathway proteins in each group were analyzed.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 24.0 software was used to analyze the data. The measurement data were expressed by (independent x±s). Independent sample t-test was used for comparison between groups, and single factor analysis of variance was used for comparison between groups. The difference was statistically significant (p<0.05).
Results and Discussion
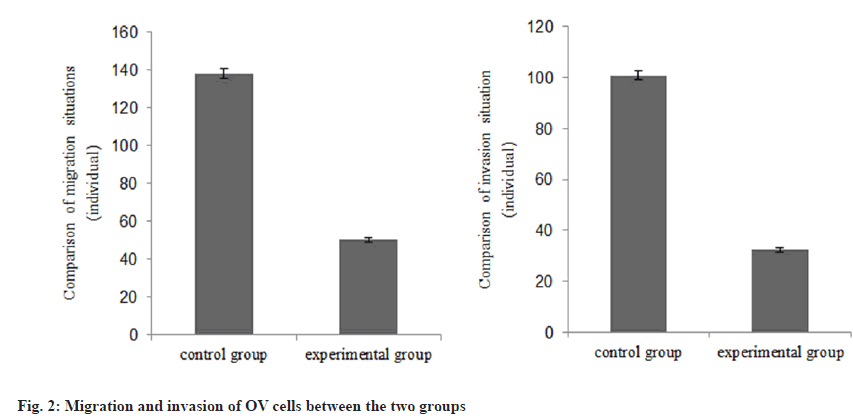
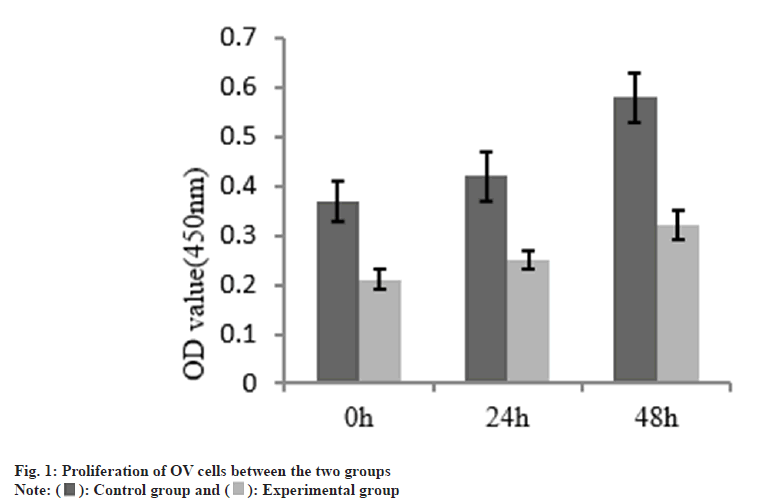
The cell proliferation in the group B was decreased than group A as shown in Table 1 and fig. 1. The number of migration and invasion of OV cells in the group B was reduced than group A as shown in Table 2 and fig. 2.
| Group | n | OD value (450 nm) | ||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| A | 3 | 0.37±0.04 | 0.42±0.05 | 0.58±0.05 |
| B | 3 | 0.21±0.02 | 0.25±0.02 | 0.32±0.03 |
| t | 5.06 | 4.464 | 6.306 | |
| p | 0.038 | 0.047 | 0.024 | |
Table 1: The Proliferation of OV Cells Between the Two Groups
| Group | n | Number of migration | Number of invasion |
|---|---|---|---|
| A | 3 | 137.84±2.47 | 100.78±1.86 |
| B | 3 | 50.15±1.28 | 32.41±0.85 |
| t | 54.596 | 57.907 | |
| p | <0.001 | <0.001 |
Table 2: Migration and Invasion of OV Cells (X̄±S)
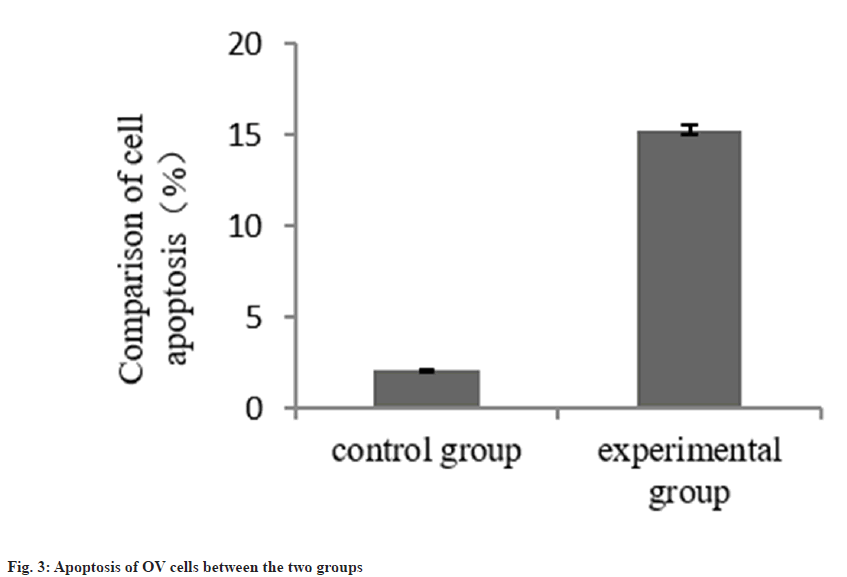
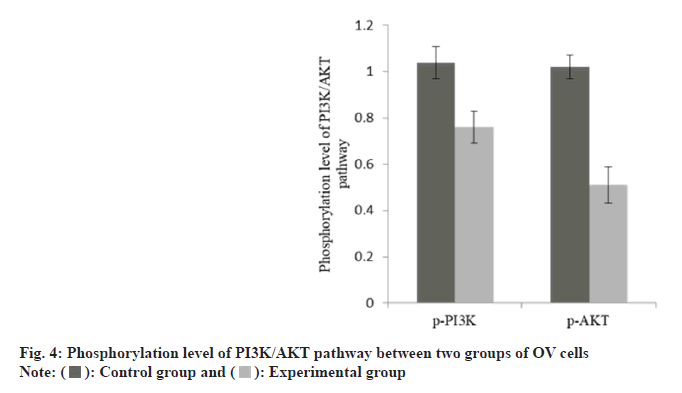
The apoptosis rate of OV cells in the group B was increased than group A as shown in Table 3 and fig. 3. The p-PI3K and p-AKT protein in the group B was decreased than group A as shown in Table 4 and fig. 4.
| Group | n | Cell apoptosis |
|---|---|---|
| A | 3 | 2.07±0.08 |
| B | 3 | 15.27±0.24 |
| t | 90.374 | |
| p | <0.001 |
Table 3: Apoptosis of OV Cells (%)
| Group | n | p-PI3K | p-AKT |
|---|---|---|---|
| A | 3 | 1.04±0.07 | 1.02±0.05 |
| B | 3 | 0.76±0.07 | 0.51±0.08 |
| t | 28.14 | 29.329 | |
| p | <0.001 | <0.001 |
Table 4: Phosphorylation Level of PI3K/AKT Pathway
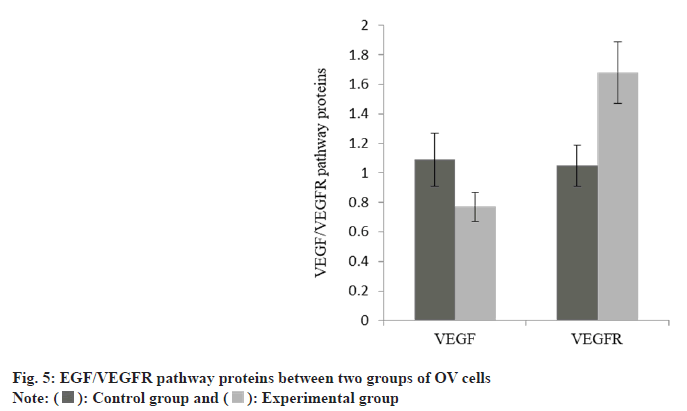
The expression of VEGF in OV cells in the group A was down-regulated, while the VEGFR was increased than group A as shown in Table 5 and fig. 5.
| Group | n | VEGF | VEGFR |
|---|---|---|---|
| A | 3 | 1.09±0.18 | 1.05±0.14 |
| B | 3 | 0.77±0.10 | 1.68±0.21 |
| t | 3.174 | 4.526 | |
| p | 0.034 | 0.004 |
Table 5: EGF/VEGFR Pathway Proteins in OV Cells
The most important biological characteristics of OV are infiltration and implantation metastasis to the surrounding tissue. 70 % of OV patients have abdominal metastasis when they are diagnosed, and the vast majority of patients will die of complications caused by metastatic foci. Therefore, effective prevention and treatment of tumor cell invasion and metastasis of OV is the focus of the current research[7,8].
VEGF promotes the oral squamous cell carcinoma behavior[9], involved in the regulation of endometrial cancer cell migration, invasion and angiogenesis[10], and inhibited migration and invasion of human hepatocellular carcinoma cell line LM3[11]. It can be seen that VEGF is highly expressed in many kinds of malignant tumors, which can promote the malignant migration and invasion of tumor cells and promote angiogenesis. At the same time, it is involved in the invasion and apoptosis of OV cells[12,13]. As a specific targeting drug, bevacizumab inhibits the proliferation, migration and invasion of tumor cells, regulates angiogenesis and inhibits tumor progression by inhibiting its specific binding to VEGFR[14]. Here, the expression of VEGF in OV cells in the group B was down-regulated. At the same time, it was found that the proliferation of OV cells in the group B was reduced than group A. The results of Transwell detection showed that the migration and invasion ability in the group B was reduced than group A. It is suggested that the anti-tumor effect of bevacizumab is achieved by inhibiting the expression of VEGF. Bevacizumab inhibits the activity of VEGF/VEGFR and down-regulates the VEGF protein through competitive binding with VEGFR, thus inhibiting the invasion, migration and angiogenesis of OV, which is consistent with the results in other tumor cells[15].
p85 and p110 are the regulatory and catalytic subunits of PI3K. When activated, inositol 3-Akt-5-phosphatidylinositol 5-triphosphate is produced. Its activation can lead to a series of Aktdependent or Akt-independent signal transduction and promote tumor cell proliferation. As one of the downstream target molecules of PI3K, the increase of Akt phosphorylation level can lead to the activation of PI3K/Akt pathway and activate the anti-apoptosis mechanism, glucose metabolism and protein synthesis of tumor cells. Activated PI3K/Akt can activate its downstream molecules through TSC1/2 complex, promote abnormal angiogenesis and participate in the regulation of malignant progression of tumor cells[16]. The p-PI3K/p-Akt protein in the group B was reduced than group A. The amount of apoptosis of OV cells in the group B was increased. It is suggested that bevacizumab can promote the apoptosis of OV cells by inhibiting the activation of PI3K/Akt phosphorylation.
To sum up, bevacizumab inhibits the malignant biological characteristics of OV by regulating VEGF/VEGFR and PI3K/Akt pathways. This study shows that bevacizumab not only specifically blocks the binding of VEGF and VEGFR, but also inhibits the malignant progression of OV by reducing VEGF expression and inhibiting PI3K/ Akt phosphorylation activation.
Conflict of interests:
The authors declared no conflict of interests.
References
- Li P, Sun H, Li W, Wu Q, Ye S, Zhu J, et al. Isolation and purification of 12 flavonoid glycosides from Ginkgo biloba extract using sephadex LH-20 and preparative high-performance liquid chromatography. Z Naturforsch C J Biosci 2022;78(1-2):73-81.
[Crossref] [Google Scholar] [PubMed]
- Lampert EJ, Zimmer A, Padget M, Cimino-Mathews A, Nair JR, Liu Y, et al. Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: A proof-of-concept phase II study. Clin Cancer Res 2020;26(16):4268-79.
[Crossref] [Google Scholar] [PubMed]
- Liu QW, Ying YM, Zhou JX, Zhang WJ, Liu ZX, Jia BB, et al. Human amniotic mesenchymal stem cells-derived IGFBP-3, DKK-3 and DKK-1 attenuate liver fibrosis through inhibiting hepatic stellate cell activation by blocking Wnt/β-catenin signaling pathway in mice. Stem Cell Res Ther 2022;13(1):224.
[Crossref] [Google Scholar] [PubMed]
- Cao Y, Ye Q, Zhuang M, Xie S, Zhong R, Cui J, et al. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PloS One 2017;12(11):e0186520.
[Crossref] [Google Scholar] [PubMed]
- Kazerounian S, Lawler J. Integration of pro-and anti-angiogenic signals by endothelial cells. J Cell Commun Signal 2018;12(1):171-9.
[Crossref] [Google Scholar] [PubMed]
- Komi Y, Suzuki Y, Shimamura M, Kajimoto S, Nakajo S, Masuda M, et al K. Mechanism of inhibition of tumor angiogenesis by β-hydroxyisovalerylshikonin. Cancer Sci 2009;100(2):269-77.
[Crossref] [Google Scholar] [PubMed]
- Su W, Hou X, Yu B. Value of dynamic contrast-enhanced magnetic resonance imaging in combination with mammography for screening early-stage breast cancer. Afr Health Sci 2023;23(2):290-7.
[Crossref] [Google Scholar] [PubMed]
- Dong T, Yuan Y, Xiang X, Sang S, Shen H, Wang L, et al. High cytoplasmic YAP1 expression predicts a poor prognosis in patients with colorectal cancer. PeerJ 2020;8:e10397.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Xu H, Sun G, Zhang Y. LncRNA CASC9 affects cell proliferation, migration and invasion of tongue squamous cell carcinoma via regulating miR-423-5p/SOX12 axes. Cancer Manag Res 2020;12:277-87.
[Crossref] [Google Scholar] [PubMed]
- Masoud GN, Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015;5(5):378-89.
[Crossref] [Google Scholar] [PubMed]
- Lai JC, Zheng YH, Tang YH. Lenalidomide inhibits the migration and invasion of human hepatoma cell line LM3 by inhibiting the expression of VEGF. J Hepatobiliary Pancreat Surg 2021;33(5):292-7.
- Gong Q, Liu C, Wang C, Zhuang L, Zhang L, Wang X. Effect of silencing TEM8 gene on proliferation, apoptosis, migration and invasion of XWLC-05 lung cancer cells. Mol Med Rep 2018;17(1):911-7.
[Google Scholar] [PubMed]
- Cui R, Wang Y, Li Y, Li Y. Clinical value of ROMA index in diagnosis of ovarian cancer: Meta-analysis. Cancer Manag Res 2019:2545-51.
[Crossref] [Google Scholar] [PubMed]
- Tu X, Zhang J, Yuan W, Wu X, Xu Z, Qing C. Simvastatin enhanced anti-tumor effects of bevacizumab against lung adenocarcinoma A549 cells via abating HIF-1α-Wnt/β-catenin signaling pathway. Anticancer Agents Med Chem 2023;23(19):2083-94.
[Crossref] [Google Scholar] [PubMed]
- Ma RJ, Kannan M, Xia Q, Zhang SS, Tu PF, Liu KC, et al. Kunxian capsule extract inhibits angiogenesis in zebrafish embryos via PI3K/AKT-MAPK-VEGF pathway. Chin J Integr Med 2023;29(2):137-45.
[Crossref] [Google Scholar] [PubMed]
- Mathews ES, Appel B. Cholesterol biosynthesis supports myelin gene expression and axon ensheathment through modulation of P13K/Akt/mTOR signaling. J Neurosci 2016;36(29):7628-39.
[Crossref] [Google Scholar] [PubMed]
 ): Control group and (
): Control group and ( ): Experimental group
): Experimental group