- *Corresponding Author:
- Lie Lin
Department of Hematology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Xiuying, Haikou 570311, China
E-mail: fxj1319459744@126.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “332-336” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the effectiveness of rituximab in conjunction with cyclosporine in managing refractory idiopathic thrombocytopenia. A retrospective analysis was performed on 210 adult individuals diagnosed with refractory idiopathic thrombocytopenia upon admission to our medical facility in 2022. Subsequently, they were categorized into distinct treatment cohorts: 98 individuals in the control group and 112 individuals in the observation group. Standard treatment protocols were administered to the control group, while the observation group received supplementary low-dose rituximab alongside the standard treatment regimen followed by the control group. The evaluation encompassed pre- and post-treatment efficacy, fluctuations in cluster of differentiate 20+ expression and platelet count, levels of interleukin-18 and tumor necrosis factor alpha in patient serum, adverse reactions, and recurrence of thrombocytopenia. 4 w post-treatment, the observation group yielded remarkably superior outcomes, with a total effective rate of 92.86 %, compared to the control group’s rate of 77.55 % (p<0.05). Moreover, the observation group demonstrated diminished cluster of differentiate 20+ expression levels and elevated platelet counts in peripheral lymphocytes relative to the control group, with notable significance (p<0.05). Subsequent to treatment, the observation group manifested diminished interleukin-18 and tumor necrosis factor alpha levels relative to the control group, with substantial significance (p<0.01). Conversely, no noteworthy contrast in the prevalence of adverse reactions was identified between the two groups (p>0.05). The recurrence rate of thrombocytopenic purpura in the observation group was notably reduced as opposed to the control group, a difference that proved to be substantially significant (p<0.05). The concurrent usage of rituximab and cyclosporine in managing refractory idiopathic thrombocytopenic purpura exhibits marked clinical effectiveness and a commendable safety profile. The integrated treatment strategy holds potential benefits for symptom amelioration, enhancement of platelet levels, and the prevention of disease relapse, indicative of favorable attributes.
Keywords
Rituximab, cyclosporine, idiopathic thrombocytopenia, clinical efficacy, glucocorticoids
Idiopathic Thrombocytopenia (ITP), also recognized as primary immune thrombocytopenia, is a hematologic condition typified by reduced platelet levels in the peripheral blood and bleeding manifestations in the skin or mucosal membranes, attributed to immunemediated mechanisms[1-3]. Despite the effective control of the majority of patient’s conditions with traditional interventions such as glucocorticoids and immunoglobulins, roughly 10 %-15 % of patients encounter refractory ITP, reflecting the ineffectiveness or variable efficacy of standard treatments[4-6]. Consequently, individuals experience persistent thrombocytopenia and recurring bleeding manifestations, profoundly impacting their overall well-being and physiological function[7-9].
Prior investigations have revealed that individual use of rituximab or Cyclosporine (CsA) has exhibited some effectiveness in addressing refractory ITP; however, their clinical utility remains somewhat constrained[10-12]. Rituximab is capable of engaging with platelet Fc-Gamma (Fcγ) receptors, fostering platelet eradication and removal, yet its effectiveness can be erratic, and severe adverse reactions may occur in certain patients[13,14]. CsA, frequently employed as an immunosuppressive agent, functions by suppressing T lymphocyte activity and modulating immune system functionality, yet its clinical applications are confronted with difficulties related to dose surveillance and potential adverse reactions[15].
In light of the limitations linked to the solo administration of rituximab or CsA, there has been a burgeoning focus on the combination of these medications to address refractory ITP. The combined regimen involving rituximab and CsA holds promise for leveraging synergistic mechanisms to bolster efficacy and curtail adverse effects in managing refractory ITP. Presently, there is a paucity of extensive research appraising the clinical effectiveness of combined rituximab and CsA therapy for refractory ITP. As a result, this study endeavors to assess the clinical effectiveness of rituximab in conjunction with CsA in treating refractory ITP, with a view to introducing novel insights and strategies for the clinical management of refractory ITP.
Materials and Methods
Clinical data:
A retrospective investigation involved 210 adult patients with refractory ITP admitted to our institution in 2022, categorized into a control (98 cases) and an observation (112 cases) group predicated on varying therapeutic approaches. The control group included 42 male and 56 female participants, aged 40 y to 57 y (51.42±5.33) y. The disease duration ranged from 3 y to 5 y (4.64±1.23) y. In comparison, the observation group encompassed 49 male and 63 female participants, aged 42 y to 60 y (50.27±5.24) y. The disease duration ranged from 3 y to 6 y (4.38±1.35) y. Remarkably, there were no substantial variances in the general characteristics of the groups (p>0.05).
Inclusion criteria: Diagnosed with refractory ITP aged 40 y to 60 y, with recurrent symptoms following splenectomy or lack of response to this procedure, ineffective glucocorticoid therapy or dependency, and failure of immunoglobulin and Thrombopoietin (TPO) therapy.
Exclusion criteria: Pregnant or lactating women; individuals with psychiatric disorders; individuals with severe liver, kidney, or cardiovascular diseases; individuals with hepatitis B or hepatitis C and individuals with Human Immunodeficiency Virus (HIV) infection.
Methods:
Standard therapeutic measures were implemented in both cohorts, entailing procedures for hemostasis, trauma prevention, enforced bed rest, and infection control[5]. Specifically within the control group, CsA therapy was administered, commencing at a 5 mg/ kg/d dosage to maintain a drug concentration within the 200-400 μg/l range. The therapeutic regimen was sustained for a period of 3 mo, and in cases of efficacy, was prolonged beyond 1 y. As an adjunct to the control group’s therapeutic protocol, the observation group underwent supplementary low-dose rituximab injections. Each administration comprised 100 mg of rituximab and transpired on a weekly basis over a 2 h period. Preceding the treatment, patients received 5 mg of dexamethasone for 3 consecutive days to preempt allergic reactions, totaling 4 administrations before cessation.
Observation indicators:
Evaluation was done based on three categories; marked effective, effective, and ineffective. If platelet count ≥100×109/l after treatment with absence of bleeding symptoms, then it was considered as markedly effective. If platelet count ≥30×109/l after treatment with minimal or no bleeding symptoms, then it was considered as effective. And if the platelet count <30×109/l after treatment with no improvement or worsening of bleeding symptoms, then it was considered ineffective. The total effective rate was calculated as the aggregate of the markedly effective rate and the effective rate.
Pre-treatment, 2 w, and 4 w post-treatment fasting venous blood specimens were procured to monitor alterations in Cluster of Differentiation 20+ (CD20+) expression and platelet count within both cohorts.
Upon conclusion of the treatment period, venous blood samples were acquired from the individuals, and the resulting serum, obtained through centrifugation, underwent enzymatic analysis using established Enzyme-Linked Immunosorbent Assay (ELISA) methods to determine Tumor Necrosis Factor-Alpha (TNF-α) and Interleukin-18 (IL-18) levels in the samples.
Adverse reactions such as headache, chest pain, liver or kidney dysfunction, fever, rash, etc., were recorded. Subsequent to the administration of diverse drug therapies, the patients were subjected to a 1 y follow-up to evaluate fluctuations in platelet count and to compare the recurrence patterns of thrombocytopenia.
Statistical analysis:
The analysis compared the measurement data between the two groups utilizing the Statistical Package for the Social Sciences (SPSS) 25.0 software, presented as mean±standard deviation, and employed the Chisquare (χ2) test for analyzing the count data, with notable significance defined at p<0.05.
Results and Discussion
Following treatment, the total effective rate in the observation group stood at 92.86 %, a substantial increase in comparison to the total effective rate of 77.55 % in the control group (p<0.05) (Table 1).
| Group (n) | Markedly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|
| Observation (112) | 55 (49.11) | 49 (43.75) | 8 (7.14) | 104 (92.86) |
| Control (98) | 41 (41.84) | 35 (35.71) | 22 (22.45) | 76 (77.55) |
| χ2 | 10.000 | |||
| p | 0.002 |
Table 1: Curative Effect (%)
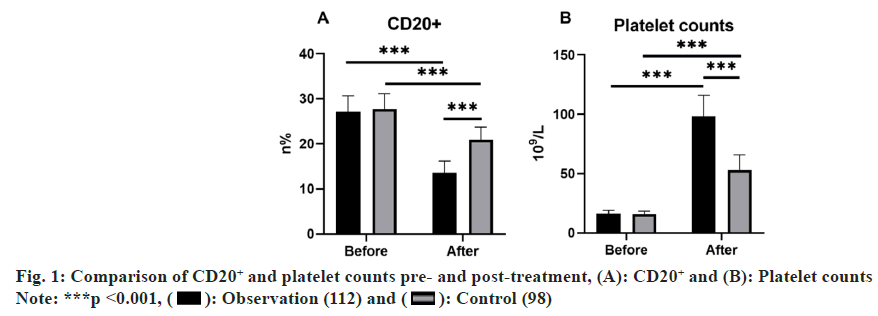
Initial CD20+ levels and platelet count measurements in peripheral lymphocytes revealed no noteworthy disparities between groups prior to treatment (p>0.05). In contrast, following a 4 w treatment, the observation group exhibited reduced CD20+ expression in peripheral lymphocytes and heightened platelet counts as relative to the control group, indicating marked distinctions (p<0.05) (fig. 1).
Preceding treatment onset, no notable variances were observed in serum IL-18 and TNF-α levels between groups (p>0.05). Nevertheless, post a 4 w treatment period, the observation group displayed diminished IL- 18 and TNF-α levels in contrast to the control group, highlighting remarkable contrasts (p<0.01) (Table 2).
| Group (n) | IL-18 | TNF-α | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Observation (112) | 281.30±26.80 | 204.87±23.83 | 76.02±8.34 | 55.49±6.35 |
| Control (98) | 282.07±30.73 | 239.80±27.27 | 74.68±8.17 | 64.34±6.32 |
| t | 0.193 | 9.905 | -1.172 | 10.101 |
| p | 0.847 | 0.000 | 0.242 | 0.000 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 2: Inflammatory Cytokines (x±s, μg/l)
The control group exhibited adverse reactions at a rate of 10.20 %, including 2 cases of chest pain, 1 case of rash, 4 cases of nausea, and 3 cases of liver and kidney dysfunction. Conversely, the observation group demonstrated a 6.25 % incidence rate, with occurrences comprising 1 case of chest pain, 2 cases of rash, 2 cases of nausea, and 2 cases of liver and kidney dysfunction. No notable difference in the incidence of adverse reactions was observed between groups (χ2=1.098, p=0.295) (Table 3).
| Group (n) | Chest pain | Rash | Nausea | Liver and kidney function damage | Overall incidence |
|---|---|---|---|---|---|
| Observation (112) | 1 (0.89) | 2 (1.79) | 2 (1.79) | 2 (1.79) | 7 (6.25) |
| Control (98) | 2 (2.04) | 1 (1.02) | 4 (4.08) | 3 (3.06) | 10 (10.20) |
| χ2 | 1.098 | ||||
| p | 0.295 |
Table 3: Adverse Reactions (n (%))
Following treatment, a 1 y tracking period revealed a substantially diminished recurrence rate of thrombocytopenic purpura in the observation group (4.46 %) as relative to the control group (19.39 %), indicating a marked statistical contrast (p<0.05) as shown in Table 4.
| Group (n) | Number of relapses | Recurrence rate |
|---|---|---|
| Observation (112) | 5 | 4.46 |
| Control (98) | 19 | 19.39 |
| χ2 | 11.499 | |
| p | 0.001 |
Table 4: Recurrence Rate (n (%))
ITP is an acquired autoimmune bleeding disorder, accounting for about 1/3rd of all bleeding disorders[16]. The primary disease mechanism stems from the patient’s immune system intolerance to self-antigens, culminating in heightened immune-mediated platelet destruction and compromised immune-mediated platelet generation by megakaryocytes[17]. The clinical profile primarily encompasses indications of cutaneous and mucosal bleeding and, in severe scenarios, may result in internal hemorrhaging, inclusive of intracranial hemorrhage[18]. Patients frequently exhibit propensities for the onset of refractory ITP even subsequent to standard treatment approaches[19]. Consequently, it is imperative to promptly commence second-line therapy for patients with refractory ITP unresponsive to steroid therapy, reliant on steroids, and exhibiting enduringly low platelet counts coupled with persistent bleeding post-splenectomy.
This research sought to assess the effectiveness of a concurrent regimen encompassing rituximab and CsA in addressing refractory ITP. The study’s outcomes revealed a substantial elevation in the overall effective rate within the observation group as relative to the control group following treatment. Post-treatment, the observation group exhibited decreased CD20+ expression levels in peripheral lymphocytes in contrast to the control group, coupled with higher platelet counts, signifying notable statistical differences. Following treatment, the levels of IL-18 and TNF-α exhibited a notable reduction in the observation group as contrasted with the control group. The frequency of adverse reactions in the observation group was marginally less than that in the control group, although this contrast lacked statistical significance. However, the recurrence rate of thrombocytopenic purpura in the observation group exhibited a noteworthy decrease as relative to the control group.
These results point to the potential advantages of employing rituximab in conjunction with CsA in treating refractory ITP. Rituximab attaches to Fc receptors on effector cells, facilitating complementdependent cytotoxicity and antibody-dependent cytotoxicity. This process culminates in the elimination of activated B-cells in blood, bone marrow, and lymph nodes, subsequently curbing autoantibody production, thereby reducing platelet destruction and enhancing platelet counts. CsA exerts selective inhibition of CD4+ cell activity, fostering a milieu conducive to the multiplication of CD8+ cells by impeding the differentiation of cytotoxic T cells, and curtailing the synthesis and release of IL-2. Furthermore, this inhibits the functionality of T helper cells, heightens the suppressive impact of CD8+ cells on B cells, lessens the generation of platelet antibodies by B cells, and diminishes platelet destruction, thereby bolstering platelet counts. The concurrent utilization of these two therapeutics can elicit synergistic effects through a dual mechanism, thereby amplifying their efficacy. The notably augmented overall effective rate observed in the observation group within this study underscores the substantial clinical effectiveness of rituximab in combination with CsA. The diminished CD20+ expression levels in peripheral lymphocytes and the augmented platelet count in the observation group relative to the control group signify a propitious impact of the combined therapeutic regimen on platelet recuperation. Furthermore, the substantial reduction in IL-18 and TNF-α levels in the observation group suggests the potential of rituximab in conjunction with CsA to attenuate the immune and inflammatory responses. An evaluation of safety revealed a marginally lower occurrence of adverse reactions in the observation group relative to the control group, albeit lacking statistical significance. Despite this, the results indicate that the utilization of rituximab in combination with CsA demonstrated a relative degree of safety within this study. Additionally, the noteworthy decrease in the recurrence rate of thrombocytopenic purpura within the observation group highlights the protracted disease control capabilities of the combined treatment. The results from the 1 y follow-up corroborate the effectiveness of rituximab combined with CsA treatment in forestalling the reappearance of thrombocytopenic purpura.
Nevertheless, it’s essential to recognize the constraints of this research. Firstly, its retrospective nature impedes the adoption of a randomized controlled and doubleblinded approach. Additionally, the evaluation solely examined post-treatment effectiveness and recurrence over a 1 y period, warranting longer-term monitoring and observation for comprehensive efficacy and safety appraisal.
In summary, the implementation of rituximab along with CsA for the treatment of refractory ITP showcased significant clinical efficacy and a relatively commendable safety profile within this study. Nevertheless, further randomized controlled trials essential to corroborate these outcomes and conduct an in-depth evaluation of the sustained efficacy and potential adverse effects. The results of this study are anticipated to introduce innovative treatment approaches for the clinical management of refractory ITP, ultimately augmenting patient’s quality of life and long-term prognosis.
Funding:
This work was supported by Hainan Provincial Natural Science Foundation of China (No: 820MS137).
Conflict of interests:
The authors declared no conflict of interests.
References
- Liu XG, Hou Y, Hou M. How we treat primary immune thrombocytopenia in adults. J Hematol Oncol 2023;16(1):4.
- Piel-Julian ML, Mahevas M, Germain J, Languille L, Comont T, Lapeyre-Mestre M, et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost 2018;16(9):1830-42.
[Crossref] [Google Scholar] [PubMed]
- Onisai M, Vladareanu AM, Spinu A, Gaman M, Bumbea H. Idiopathic thrombocytopenic purpura (ITP)-New era for an old disease. Rom J Intern Med 2019;57(4):273-83.
[Crossref] [Google Scholar] [PubMed]
- Sandal R, Mishra K, Jandial A, Sahu KK, Siddiqui AD. Update on diagnosis and treatment of immune thrombocytopenia. Exp Rev Clin Pharmacol 2021;14(5):553-68.
[Crossref] [Google Scholar] [PubMed]
- Dou X, Yang R. Current and emerging treatments for immune thrombocytopenia. Exp Rev Hematol 2019;12(9):723-32.
[Crossref] [Google Scholar] [PubMed]
- Lv Y, Shi H, Liu H, Zhou L. Current therapeutic strategies and perspectives in refractory ITP: What have we learned recently? Front Immunol 2022;13:953716.
[Crossref] [Google Scholar] [PubMed]
- Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 2019;3(23):3829-66.
[Crossref] [Google Scholar] [PubMed]
- Kohli R, Chaturvedi S. Epidemiology and clinical manifestations of immune thrombocytopenia. Hämostaseologie 2019;39(03):238-49.
[Crossref] [Google Scholar] [PubMed]
- Rajesh R, Shanmugam MP, Sagar P. Idiopathic thrombocytopenic purpura and its fundus features in a patient with diabetes mellitus. Indian J Ophthalmol 2020;68(11):2587-9.
[Crossref] [Google Scholar] [PubMed]
- Delrue M, Baylatry MT, Joly AC, Corre E, Marjanovic Z, El-Khoury-Hanna N, et al. Efficacy of subcutaneous preemptive rituximab in immune-mediated thrombotic thrombocytopenic purpura: Experience from the first 12 cases. Am J Hematol 2021;96(1):E26-9.
[Crossref] [Google Scholar] [PubMed]
- Wu YJ, Liu H, Zeng QZ, Liu Y, Wang JW, Wang WS, et al. All-trans retinoic acid plus low-dose rituximab vs. low-dose rituximab in corticosteroid-resistant or relapsed ITP. Blood 2022;139(3):333-42.
[Crossref] [Google Scholar] [PubMed]
- Mousavi-Hasanzadeh M, Bagheri B, Mehrabi S, Eghbali A, Eghbali A. Sirolimus vs. cyclosporine for the treatment of pediatric chronic immune thrombocytopenia: A randomized blinded trial. Int Immunopharmacol 2020;88:106895.
[Crossref] [Google Scholar] [PubMed]
- Galvez-Cancino F, Simpson AP, Costoya C, Matos I, Qian D, Peggs KS, et al. Fcγ receptors and immunomodulatory antibodies in cancer. Nat Rev Cancer 2024;24(1):51-71.
- Schmidt DE, de Haan N, Sonneveld ME, Porcelijn L, van der Schoot CE, de Haas M, et al. IgG-Fc glycosylation before and after rituximab treatment in immune thrombocytopenia. Sci Rep 2020;10(1):3051.
[Crossref] [Google Scholar] [PubMed]
- Ito M, Yagasaki H, Kanezawa K, Shimozawa K, Hirai M, Morioka I. Incidence and outcomes of refractory immune thrombocytopenic purpura in children: A retrospective study in a single institution. Sci Rep 2021;11(1):14263.
[Crossref] [Google Scholar] [PubMed]
- Mithoowani S, Cervi A, Shah N, Ejaz R, Sirotich E, Barty R, et al. Management of major bleeds in patients with immune thrombocytopenia. J Thromb Haemost 2020;18(7):1783-90.
[Crossref] [Google Scholar] [PubMed]
- LeVine DN, Brooks MB. Immune thrombocytopenia (ITP): Pathophysiology update and diagnostic dilemmas. Vet Clin Pathol 2019;48(1):17-28.
[Crossref] [Google Scholar] [PubMed]
- Connors JM, Fein S. How to manage ITP with life-threatening bleeding. Hematol 2023;2023(1):254-8.
[Crossref] [Google Scholar] [PubMed]
- Vernava I, Schmitt CA. Daratumumab as a novel treatment option in refractory ITP. Blood Cells Mol Dis 2023;99:102724.
[Crossref] [Google Scholar] [PubMed]