- *Corresponding Author:
- Jinjin Zhan
Department of Geriatric Medicine, The Fourth Medical Center of People’s Liberation Army (PLA) General Hospital, Haidian, Beijing 100039, China
E-mail: Zjjin2021@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “303-309” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To determine the effectiveness of nicorandil in conjunction with trimetazidine on elderly individuals with coronary heart disease-induced chronic heart failure and its influence on the cardiac function. Between January 2018 and December 2022, a retrospective analysis was performed on the data of 248 elderly individuals with coronary heart disease-induced chronic heart failure treated in our hospital. All patients received routine basic treatment, with 110 patients treated with trimetazidine assigned to the control group, and the remaining 138 patients receiving both nicorandil and trimetazidine assigned to the study group. Prior to and after treatment, an evaluation of the cardiac function-related parameters (including left ventricular ejection fraction, left ventricular end-diastolic dimension, and N-terminal pro-brain natriuretic peptide) as well as inflammatory factors (C-reactive protein and interleukin-6) was performed and compared between the two groups. A comparison was made between the two groups regarding efficacy and adverse reactions, followed by logistic regression analysis to examine the risk factors affecting the patient’s prognosis. Risk factors affecting the prognosis of patients, as identified by univariate analysis, included age, course of coronary heart disease, New York heart association classification, history of smoking, history of alcoholism, and medication regimen. The prognosis of individuals was found to be influenced by independent risk factors, namely New York heart association classification and medication regimen, as determined by multivariate logistic regression analysis. Effectively treating elderly individuals with coronary heart disease-induced chronic heart failure involves the utilization of nicorandil and trimetazidine in combination. This method not only promotes cardiac function recovery and enhances myocardial cells, but also mitigates inflammatory reactions, all while avoiding the occurrence of increased adverse reactions. Moreover, the prognosis of individuals was influenced by independent risk factors, namely New York heart association classification and medication regimen.
Keywords
Nicorandil, trimetazidine, coronary heart disease, chronic heart failure, cardiac function
Coronary Heart Disease (CHD) is a prevalent cardiovascular disorder among the aged population, which is mainly triggered by the imbalance of myocardial energy metabolism, the low pumping function of ventricle and the impaired myocardial structure and function due to long-term hypoxia and ischemia of coronary artery[1,2]. Heart failure is a major complication of CHD[3]. In elderly patients with CHDinduced Chronic Heart Failure (CHF), symptoms predominantly arise from an elevation in hemodynamic load, which will damage myocardial structure, cardiac function and myocardium, and the symptoms include dyspnea, fatigue and urinary retention, which seriously disrupt the normal life of patients[4,5].
At the current stage, CHD-induced CHF is mainly treated by drugs, which is to delay the deterioration of cardiac function[6]. Nicorandil is an ATP-sensitive potassium channel opener, with the functions of dilating blood vessels, increasing blood flow and reducing heart load, which is conducive to relaxing blood vessels and smoothing muscle, as well as taking effects in anti-inflammation, stabilizing plaque and improving endothelial function[7,8]. Trimetazidine is a kind of myocardial energy metabolism drug, which can relieve the inflammatory reaction and protect the myocardium[9]. The effect of a single drug cannot meet the clinical expectation, but there are still few studies on nicorandil in conjunction with trimetazidine in managing elderly individuals with CHD-induced CHF.
Accordingly, this study explored the effectiveness of nicorandil conjunction with trimetazidine in managing elderly individuals with CHD-induced CHF and its impact on the cardiac function, with the goal of offering evidence-based recommendations for the management of this disease.
Materials and Methods
Sample information:
Between January 2018 and December 2022, a retrospective analysis was performed on the data of 248 elderly individuals with CHD-induced CHF treated in our hospital.
Ethical statement:
The Medical Ethics Committee of our hospital granted permission for the implementation of this study.
Inclusion and exclusion criteria:
Inclusion criteria: Individuals who fulfilled the diagnostic criteria of CHD-induced CHF were; typical angina pectoris, myocardial ischemia, orthopnea, paroxysmal nocturnal dyspnea, chest distress, shortness of breath, palpitation, abdominal distension, enlarged border of cardiac dullness, dry and wet rales in both lungs, engorgement of the neck veins, a positive hepatojugular reflux sign, peripheral edema, etc.,[4] and were diagnosed as CHD-induced CHF by comprehensive judgment such as electrocardiogram and echocardiography; individuals who were aged 65 or older and individuals with detailed clinical data and classified as New York Heart Association (NYHA) II-IV.
Exclusion criteria: Individuals with severe liver or kidney failure, heart failure caused by acute myocardial infarction and other reasons, bronchial asthma, severe infection or drug contraindications; patients who had received coronary artery bypass grafting or percutaneous coronary intervention recently; patients comorbid with severe diseases (such as malignant tumors, etc.,) that impact the study and individuals with allergies to the drugs administered in this study.
Sample screening:
Following the criteria-based screening process, a total of 300 patients were initially assessed, resulting in the inclusion of 248 patients who met the specified requirements for this study. All patients received routine basic treatment, with 110 patients treated with trimetazidine assigned to the control group, and the remaining 138 patients receiving both nicorandil and trimetazidine assigned to the study group.
Therapeutic regimen:
Routine medical treatment and symptomatic support, which encompassed interventions like oxygen inhalation, cardiotonic agents, Beta (β)-receptor blockers, diuretics, angiotensin converting enzyme inhibitors or angiotensin receptor antagonists, etc., were provided to patients in both groups. The control group was orally given trimetazidine dihydrochloride tablets (Les Laboratoires Servier Industries, state food and drug administration approval number: H20055465), 20 mg/time, 3 times/d. For the study group, nicorandil tablets (Nipro Pharma Corporation Kagamiishi Plant, Saudi Food and Drug Authority (SFDA) approval number: HJ20160540) were administered orally at a dosage of 5 mg for three times a day, mirroring the dosage of trimetazidine used in the control group. Both groups received treatment for a period of 3 mo.
Outcome measures:
Primary outcome measures: Left Ventricular Ejection Fraction (LVEF), Left Ventricular End- Diastolic Dimension (LVEDD), and N-Terminal pro- Brain Natriuretic Peptide (NT-pro BNP), LVEF and LVEDD were monitored by echocardiography prior to and following treatment. Fasting venous blood was acquired from each individual in the morning prior to and following treatment, and the serum was obtained by 10 min centrifugation with a low-speed centrifuge (3000 r/min). Then the level of serum NT-pro BNP was measured by Enzyme-Linked Immunosorbent Assay (ELISA). In markedly effective, the patient’s symptoms essentially vanished, accompanying a twograde improvement in cardiac function; the patient displayed relief from symptoms and showcased a one-grade improvement in cardiac function was considered as effective and in ineffective, the patient did not meet the above requirements, and even had an aggravating trend. The calculation for the overall response rate involved determining the percentage of cases that displayed marked effectiveness or effectiveness in treatment, relative to the total number of cases, multiplied by 100 %.
Secondary outcome measures: The serum levels of C-Reactive Protein (CRP) and Interleukin-6 (IL-6) were assessed using an ELISA, both prior to and following treatment; adverse reactions, such as abdominal distension, nausea and vomiting, dizziness, and fatigue, were counted and analyzed; assessment of patient efficacy was conducted, and logistic regression analysis was performed to analyze the risk factors that impacted prognosis.
Statistical analysis:
The analysis compared the measurement data between the two groups utilizing the Statistical Package for the Social Sciences (SPSS) 20.0 software, presented as mean±standard deviation, and employed the Chi-square (χ2) test for analyzing the count data, with significance defined at p<0.05. The data was visualized into desired graphs using the GraphPad 8 software package. By employing logistic regression, an assessment of the risk factors influencing the prognosis of the patients was performed.
Results and Discussion
The baseline data comparison between the two groups showed no notable variations in age, gender, Body Mass Index (BMI), duration of CHD, NYHA classification, smoking history, alcoholism history, and place of residence (p>0.05, Table 1).
| Factors | Study group (n=138) | Control group | χ2 | p | |
|---|---|---|---|---|---|
| Age | ≥70 y old | 50 | 51 | 2.603 | 0.108 |
| <70 y old | 88 | 59 | |||
| Gender | Male | 65 | 45 | 0.951 | 0.33 |
| Female | 73 | 65 | |||
| BMI | ≥23 kg/m2 | 71 | 60 | 0.236 | 0.628 |
| < 23 kg/m2 | 67 | 50 | |||
| Course of coronary heart disease | ≥5 y | 57 | 47 | 0.051 | 0.822 |
| <5 y | 81 | 63 | |||
| NYHA class | Class II-III | 86 | 65 | 0.268 | 0.605 |
| Class IV | 52 | 45 | |||
| History of smoking | Yes | 65 | 41 | 2.416 | 0.12 |
| No | 73 | 69 | |||
| History of alcoholism | Yes | 45 | 25 | 2.95 | 0.086 |
| No | 93 | 85 | |||
| Place of residence | Rural areas | 88 | 67 | 0.214 | 0.644 |
| Urban areas | 50 | 43 | |||
Table 1: Baseline data
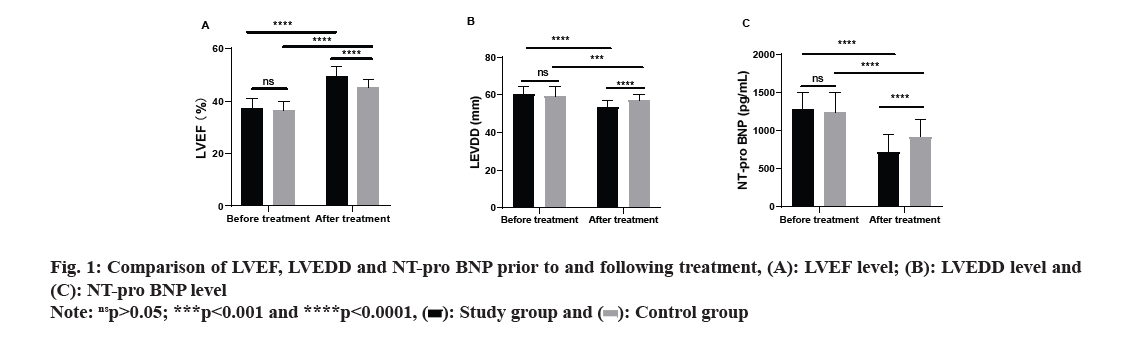
Comparing the levels of LVEF, LVEDD, and NT-pro BNP between the two groups prior to and following treatment, no remarkable differences were identified in these levels prior to treatment (p>0.05). However, following treatment, both groups saw a substantial increase in LVEF, along with a notable decrease in LVEDD and NT-pro BNP levels (p<0.05). Further analysis revealed that, following treatment, the study group exhibited a remarkably higher LVEF level (p<0.0001) and lower levels of LVEDD and NT-pro BNP as opposed to the control group (p<0.0001, fig. 1).
Prior to treatment, no noteworthy differences in CRP and IL-6 levels between the two groups was observed (p>0.05). Following treatment, both groups exhibited a considerable reduction in the levels of CRP and IL-6 (p<0.0001), with a more substantial decrease found in the study group compared to the control group (p<0.0001, fig. 2).
The analysis of clinical efficacy revealed a marked disparity in the overall response rate between the two groups, with the control group exhibiting a remarkably lower rate as opposed to the study group (p=0.003, Table 2).
| Group | Markedly effective | Effective | Ineffective | Overall response |
|---|---|---|---|---|
| Study (n=138) | 84 (60.87) | 44 (31.88) | 10 (7.25) | 128 (92.75) |
| Control (n=110) | 49 (44.55) | 39 (35.45) | 22 (20.00) | 88 (80.00) |
| χ2 | 6.559 | 0.350 | 8.859 | 8.859 |
| p | 0.010 | 0.554 | 0.003 | 0.003 |
Table 2: Comparison of efficacy (N (%))
No noteworthy difference was observed in the total incidence of adverse reactions between the two groups, as revealed by statistical analysis (p=0.853, Table 3).
| Group | Abdominal distention | Nausea and vomiting | Dizziness | Fatigue | Total adverse reaction |
|---|---|---|---|---|---|
| Study (n=138) | 3 (2.17) | 2 (1.45) | 1 (0.72) | 2 (1.45) | 8 (5.79) |
| Control (n=110) | 4 (3.64) | 1 (0.91) | 1 (0.91) | 1 (0.91) | 7 (6.34) |
| χ2 | 0.035 | ||||
| p | 0.853 |
Table 3: Incidence of adverse reactions (N (%))
Following treatment, patients who experienced marked effectiveness or effectiveness in their treatment outcome were classified as having a positive prognosis, and were subsequently assigned to the good-prognosis group (n=216). Patients who achieved an ineffective treatment outcome were categorized as having a poor prognosis, and were included in the poor-prognosis group (n=32). Univariate analysis of the clinical data from the two groups revealed that age, duration of CHD, NYHA classification, history of smoking, history of alcoholism, and medication regimen were identified as risk factors impacting the prognosis of the patients (Table 4). Subsequent to identification, the indicators that exhibited noteworthy differences were specified (Table 5) and were later examined using multivariate analysis. Independent risk factors influencing the prognosis of the patients were identified as NYHA classification and medication regimen, as per the results of multivariate logistic regression analysis (Table 6).
| Factors | Good-prognosis group (n=216) | Poor prognosis group (n=32) | χ2 | p | |
|---|---|---|---|---|---|
| Age | ≥70 y old | 78 | 23 | 14.771 | 0.0001 |
| <70 y old | 138 | 9 | |||
| Gender | Male | 93 | 17 | 1.145 | 0.285 |
| Female | 123 | 15 | |||
| BMI | ≥23 kg/m2 | 111 | 20 | 1.381 | 0.24 |
| <23 kg/m2 | 105 | 12 | |||
| Course of coronary heart disease | ≥5 y | 76 | 28 | 31.331 | <0.0001 |
| <5 y | 140 | 4 | |||
| NYHA class | Class II-III | 139 | 12 | 8.438 | 0.004 |
| Class IV | 77 | 20 | |||
| History of smoking | Yes | 85 | 21 | 7.861 | 0.005 |
| No | 131 | 11 | |||
| History of alcoholism | Yes | 45 | 25 | 16.191 | <0.0001 |
| No | 93 | 85 | |||
| Place of residence | Rural areas | 138 | 17 | 1.378 | 0.241 |
| Urban areas | 78 | 15 | |||
| Therapeutic regimen | Nicorandil+trimetazidine | 125 | 13 | 4.073 | 0.044 |
| Trimetazidine | 90 | 20 | |||
Table 4: Univariate analysis
| Factors | Assignment |
|---|---|
| Age | <70 y old=0 and ≥70 y old=1 |
| Course of coronary heart disease | <5 y=0 and ≥5 y=1 |
| NYHA classification | Class II-III=0 and class IV=1 |
| History of smoking | None=0 and yes=1 |
| History of alcoholism | None=0 and yes=1 |
| Medication regimen | Nicorandil+trimetazidine=0 and trimetazidine=1 |
| Prognosis | Good prognosis=0 and poor prognosis=1 |
Table 5: Assignment
| Factors | B | SE | Wald | Df | Sig. | Exp (B) | 95 % CI for Exp (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Age | 0.523 | 0.393 | 1.777 | 1 | 0.183 | 1.687 | 0.782 | 3.642 |
| Course of coronary heart disease | 0.333 | 0.395 | 0.709 | 1 | 0.4 | 1.395 | 0.643 | 3.026 |
| NYHA classification | -1.04 | 0.479 | 4.715 | 1 | 0.03 | 0.354 | 0.138 | 0.904 |
| History of smoking | 0.335 | 0.401 | 0.698 | 1 | 0.404 | 1.398 | 0.637 | 3.068 |
| History of alcoholism | -0.562 | 0.491 | 1.309 | 1 | 0.252 | 0.57 | 0.218 | 1.492 |
| Medication regimen | -1.083 | 0.453 | 5.703 | 1 | 0.017 | 0.339 | 0.139 | 0.824 |
Note: (SE): Standard error; (Df): Degrees of freedom; (Sig.): Significance; (Exp (B)): Exponentiation of the B coefficient and (CI): Confidence interval
Table 6: Multivariate logistic regression analysis
The final stage of CHD, known as CHF, is prevalent among the elderly population with a high incidence[10]. Without timely treatment, it will aggravate the symptoms of heart failure and even endanger the life of patients, which is not conducive to their prognosis[11]. With the increasing proportion of the elderly population in society, CHD-induced CHF has become the main reason for admission of the elderly to a hospital, which has brought huge economic burden to the society[12-14]. Therefore, its treatment is one of the hot issues in clinical research.
At the current stage, the main treatment method for CHD-induced CHF is drug therapy. Nicorandil is a nitrate drug, which can effectively treat angina pectoris of CHD, and its clinical effect is more significant than that of ordinary nitrate drugs, with relatively few adverse reactions and side effects[15-17]. Trimetazidine is a piperazine derivative, which can directly act on 3-cupric acyl-Coenzyme A (CoA) thiolytic enzyme to inhibit myocardial fat oxidation, improve myocardial oxygen consumption and thus play a protective role[18]. This study explored the efficacy of nicorandil in conjunction with trimetazidine on elderly individuals with CHDinduced CHF and the influence on the cardiac function.
LVEF, LVEDD and NT-pro BNP are all cardiac function-associated indexes, which can be adopted for evaluating and monitoring the functional state of the heart[19]. In this study, similar levels of LVEF, LVEDD and NT-pro BNP were observed prior to treatment. Post-treatment, both groups demonstrated a noticeable elevation in LVEF and decrease in LVEDD and NT-pro BNP levels, with the study group displaying a more substantial increase and reductions in comparison to the control group. The findings imply that nicorandil in conjunction with trimetazidine can improve the cardiac functionassociated indexes of patients and can take a positive role in promoting the reflux of cardiac ejection and venous blood, body remodeling and cardiac remodeling, thus effectively promoting the recovery of cardiac function and improving cardiomyocytes. CRP is a common inflammatory marker, and its high level suggests a possible intimal injury and atherosclerosis[20]. In patients with CHF, the level of IL-6, a pro-inflammatory factor, tends to increase[21]. The increase of IL-6 may be bound up with myocardial injury, ventricular remodeling and deterioration of cardiac function. Pre-treatment, the CRP and IL-6 levels in both groups were similar in this study. Subsequently, following treatment, both groups experienced a remarkable decrease in CRP and IL-6 levels, with the study group demonstrating a more pronounced decline. It implies that nicorandil in conjunction with trimetazidine can alleviate the inflammatory reaction of patients with CHD-induced CHF more effectively than trimetazidine alone. In this study, no remarkable difference was noted between the two groups in the total incidence of adverse reactions, but the control group exhibited a substantially lower overall response rate compared to the study group. The results imply that nicorandil in conjunction with trimetazidine can effectively manage patients with CHD-induced CHF without increasing adverse reactions. Wu et al.[22] have also found that nicorandil combined with trimetazidine can effectively treat patients with CHD, which align with the results of this research. The analysis of this study revealed that the factors influencing the patients’ prognosis included age, course of CHD, NYHA classification, history of smoking, history of alcoholism, and medication regimen. According to the results of the logistics regression analysis, NYHA classification and medication regimen emerged as independent risk factors influencing the patient’s prognosis.
Although the research has yielded a number of positive findings, it is not without limitations. Some variations in the study’s conclusions may be attributed to the limited sample size. Additionally, further research is necessary to determine the optimal dosages of both drugs, as the current study did not address this aspect.
In summary, nicorandil in conjunction with trimetazidine is effective in treating elderly patients with CHD-induced CHF, which can improve the recovery of the cardiac function, enhance myocardial cells and relieve inflammatory reaction, without the increase of adverse reactions. Furthermore, independent risk factors impacting patient prognosis include NYHA classification and medication regimen.
Authors’ contributions:
Lei Wang and Wenqian Wang have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bai MF, Wang X. Risk factors associated with coronary heart disease in women: A systematic review. Herz 2020;45(1):52-7.
[Crossref] [Google Scholar] [PubMed]
- Venkatachalam A, Levy J, Perolini S, Vignaux L, Mounier-Vehier C. Acute coronary heart disease and women. Rev Med Suisse 2022;18:1664-9.
- Nichols S, McGregor G, Breckon J, Ingle L. Current insights into exercise-based cardiac rehabilitation in patients with coronary heart disease and chronic heart failure. Int J Sports Med 2021;42(1):19-26.
[Crossref] [Google Scholar] [PubMed]
- Yan J, Pan Y, He Y, Wang R, Shao W, Dong S. The effects of serum iron level without anemia on long-term prognosis of patients with coronary heart disease complicated with chronic heart failure: A retrospective cohort study. Heart Vessels 2020;35:1419-28.
[Crossref] [Google Scholar] [PubMed]
- Oldridge N, Taylor RS. Cost-effectiveness of exercise therapy in patients with coronary heart disease, chronic heart failure and associated risk factors: A systematic review of economic evaluations of randomized clinical trials. Eur J Prev Cardiol 2020;27(10):1045-55.
[Crossref] [Google Scholar] [PubMed]
- Marushchak M, Krynytska IN, Fedechko MA, Sukhovolets IR, Sydorenko OK. Effects of the therapy combining calcium and vitamin D3 supplement with calcitonin on bone tissue density in patients with coronary heart disease complicated with chronic heart failure. Pol Merkur Lekarski 2019;47(280):128-33.
[Google Scholar] [PubMed]
- Du X, Ma Z, Li L, Zhong X. Nicorandil decreases renal injury in patients with coronary heart disease complicated with type I cardiorenal syndrome. J Cardiovasc Pharmacol 2021;78(5):e675-80.
[Crossref] [Google Scholar] [PubMed]
- Pang Z, Zhao W, Yao Z. Cardioprotective effects of nicorandil on coronary heart disease patients undergoing elective percutaneous coronary intervention. Med Sci Monitor 2017;23:2924-30.
[Crossref] [Google Scholar] [PubMed]
- Mou C, Mou J, He G, Yu Y. Inhibitory effects of trimetazidine on rats with coronary heart disease. Minerva Med 2020.
[Crossref] [Google Scholar] [PubMed]
- Spinar J, Spinarova L, Vitovec J. Pathophysiology, causes and epidemiology of chronic heart failure. Vnitr Lek 2018;64(9):834-8.
[Google Scholar] [PubMed]
- Vitovec J, Spinar J, Spinarova L. Betablockers after myocardial infarction and chronic coronary heart disease. Vnitr Lek 2022;68(3):178-80.
- Lu Y, Wang F, Ni H, Sun Y, Shi H. Observation of curative effect of trimetazidine combined with metoprolol in elderly patients with coronary heart disease complicated with heart failure and the effect of myocardial remodeling by integrated traditional Chinese and Western medicine. Biomed Res Int 2022;2022:6098799.
[Crossref] [Google Scholar] [PubMed]
- Lao XQ, Liu X, Deng HB, Chan TC, Ho KF, Wang F, et al. Sleep quality, sleep duration, and the risk of coronary heart disease: A prospective cohort study with 60 586 adults. J Clin Sleep Med 2018;14(1):109-17.
[Crossref] [Google Scholar] [PubMed]
- Kang X, Dong C, Liu C. Analyzation of the clinical effects of fine nursing model combined with integrated medical care intervention on elderly patients with chronic obstructive pulmonary disease. J Mod Nurs Pract Res 2021;1(3):12.
- Zeng Z, Zhang H, Zhang P, Li Y, Fu N. Standard vs. double dose of intravenous nicorandil in preventing contrast-induced nephropathy in patients with coronary heart disease undergoing elective coronary procedures. Coron Artery Dis 2021;32(3):256-7.
[Crossref] [Google Scholar] [PubMed]
- Gvishiani M, Gabunia L, Makharadze T, Gongadze N. Nicorandil efficacy in the treatment of ischemic heart disease. Georgian Med News 2018;280:152-5.
[Google Scholar] [PubMed]
- Amrutkar RD, Bhamare VG, Kapse SN. Chemogenomics: Is a promising area for drug target and discovery. J Mod Biol Drug Discov 2023;2(2).
- Mahajan S, Mahajan AU. Current clinical evidence of trimetazidine in the management of heart disease in patients with diabetes. J Assoc Physicians India 2020;68(11):46-50.
[Google Scholar] [PubMed]
- An Y, Wang Q, Wang H, Zhang N, Zhang F. Clinical significance of sFRP5, RBP-4 and NT-proBNP in patients with chronic heart failure. Am J Transl Res 2021;13(6):6305.
[Google Scholar] [PubMed]
- Castro AR, Silva SO, Soares SC. The use of high sensitivity C-reactive protein in cardiovascular disease detection. J Pharm Pharm Sci 2018;21:496-503.
[Crossref] [Google Scholar] [PubMed]
- Ptaszynska-Kopczynska K, Szpakowicz A, Marcinkiewicz-Siemion M, Lisowska A, Waszkiewicz E, Witkowski M, et al. Interleukin-6 signaling in patients with chronic heart failure treated with cardiac resynchronization therapy. Arch Med Sci 2017;13(5):1069-77.
[Crossref] [Google Scholar] [PubMed]
- Wu Y, Fan Y, Huang N, Zhang S, Zhang H, Liu X, et al. Effect of nicorandil combined with trimetazidine on miR-223-3p and NRF2 expression in patients with coronary heart disease. Am J Transl Res 2021;13(5):4804-11.
[Google Scholar] [PubMed]
 ): Study group and (
): Study group and ( ): Control group
): Control group 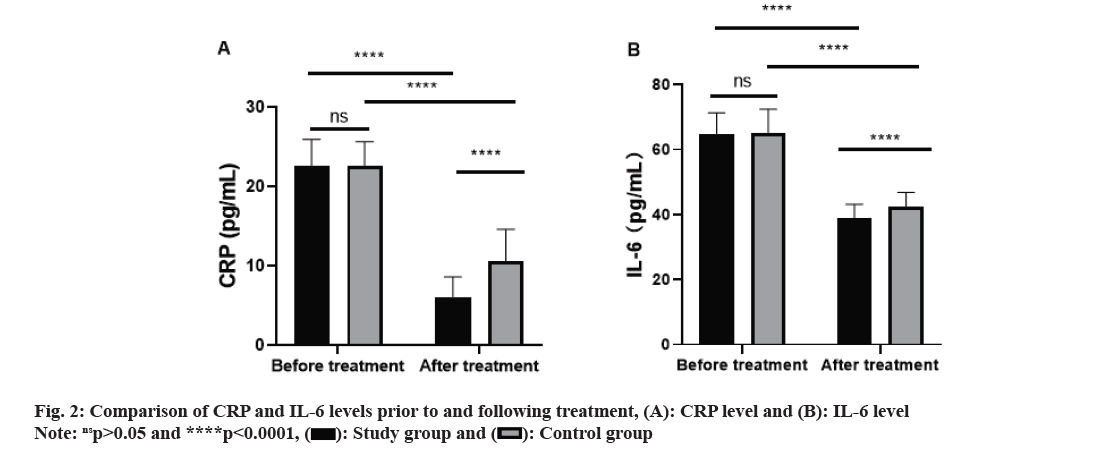
 ): Study group and (
): Study group and ( ): Control group
): Control group 



