- *Corresponding Author:
- Xinyu Lin
Department of Dermatology, Southwest Medical University, Luzhou, Sichuan 646000, China
E-mail: 15882098720@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “337-341” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the clinical effectiveness of combining hydroxychloroquine with azithromycin for managing rosacea. Our hospital conducted a retrospective investigation on 90 patients with rosacea who sought outpatient care between October 2022 and October 2023. They were divided into control arm and observation, control group was administered hydroxychloroquine and the other group received a combination of hydroxychloroquine+azithromycin. A comparison was made between the two groups regarding the duration of symptom improvement, symptom scores, psychological assessment scores, treatment results. There was remarkable decrease in the duration of clinical symptom improvement in observation compared to the control group. The observation group had better symptom scores and psychological scores after treatment compared to the control group. Combining hydroxychloroquine with azithromycin yields favorable clinical outcomes in rosacea treatment. This combined treatment regimen can significantly shorten the improvement time of clinical symptoms, improve symptom scores and psychological status, and increase the treatment success rate. The incidence of adverse reactions is relatively low. Larger-scale clinical studies and extended follow-up periods are necessary to confirm the efficacy and safety of this treatment.
Keywords
Hydroxychloroquine, azithromycin, rosacea, clinical efficacy
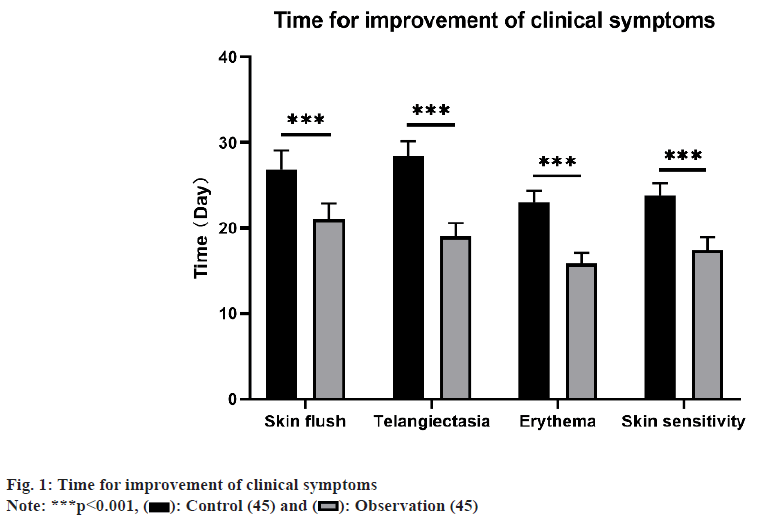
Affecting predominantly adolescents and young adults, rosacea is a persistent inflammatory ailment of the hair follicles and sebaceous glands, presenting with papules, erythema, pustules, and telangiectasia on the face, leading to a notable impact on the physical and mental health of individuals, thus diminishing their quality of life[1,2]. The etiology of this condition remains unclear; however, contemporary research points to its predominant association with deviations in innate immune function, neurovascular dysregulation, microbial infections, impaired skin barrier function, and genetic factors[3]. The treatment of rosacea includes both oral and topical medications. Expert consensus suggests that combination of multiple treatment modalities might be effective for managing the diverse symptoms of rosacea[4,5]. Hydroxychloroquine, an organic compound extensively utilized in treating malaria and rheumatic diseases[6,7], has been the subject of recent studies suggesting therapeutic impact on skin conditions. These studies propose that hydroxychloroquine may mitigate symptoms by inhibiting inflammation, modulating immune responses, and demonstrating antimicrobial effects[8,9]. In managing papules and pustules associated with rosacea, antibiotics are commonly prescribed as the first hand systemic treatment[10,11]. Azithromycin, a broad-spectrum antibiotic with antibacterial and antiinflammatory properties, is commonly used to treat skin infections and inflammations such as acne[12,13]. Nonetheless, there remains an absence of definitive research on the clinical efficacy of the combination of hydroxychloroquine with azithromycin for treating rosacea. Consequently, our research endeavors to assess the clinical efficacy of hydroxychloroquine in conjunction with azithromycin, aiming to present a more potent treatment choice for this condition. We hypothesize that this combination therapy can significantly improve the skin symptoms and disease activity in patients, thus increasing the treatment success rate and satisfaction. Overall, our study is focused on appraising the clinical efficacy of hydroxychloroquine in conjunction with azithromycin for managing rosacea, and we hope that it will offer a more effective therapeutic option for this condition. Throughout the timeframe spanning October 2022 to October 2023, a retrospective investigation was carried out involving 90 patients afflicted with rosacea who were treated as outpatients at our medical facility. Subsequently, the patients were categorized into a control group (n=45) and an observation group (n=45) based on different treatment modalities. The control group comprised 7 men and 38 women, aged between 30 y and 51 y, (37.48±3.45). The duration of illness varied from (2-36) mo, (25.33±5.34). The observation group consisted of 6 males and 39 females, with ages ranging from 31 y to 54 y, (37.24±3.64). The duration of the disease varied from (3-37) mo, (24.87±5.24). No remarkable differences in general information was identified (p>0.05), suggesting comparability. Approval for this study was obtained from the Medical Ethics Committee of our hospital. The control group received hydroxychloroquine administration (Shanghai Pharmaceuticals (Group) Co., Ltd., Approval Number: National Drug Approval Number H19990264), 200 mg per dose, twice a day, orally for 1 mo. The observation group’s treatment encompassed a regimen that included azithromycin, in addition to the therapy administered to the control group. Azithromycin tablets (Zhejiang Huaren Sanjiu Zhongyi Pharmaceutical Co., Ltd., Approval Number: National Drug Approval Number H20084458), 500 mg per dose, once a day, taken for 3 d, then discontinued for 4 d after treatment, one course lasted for 2 w, and oral administration was for 4 courses. The study compared the disparities between the two groups concerning the duration of clinical symptom improvement, rosacea symptom scores, Hamilton Anxiety Rating Scale (HAMA) scores, Hamilton Depression Rating Scale (HAMD), treatment effectiveness, and adverse reactions. Clinical symptom improvement time includes time for facial flushing, telangiectasia, erythema, and sensitive skin[4]. Appraising rosacea symptoms a comparison of patient’s clinical presentation prior to and following treatment, utilizing a scoring range of 0 (no symptoms) to 3 (severe symptoms). The HAMA, with scores reaching up to 64, where higher scores signify heightened anxiety severity. The degree of anxiety is considered severe if the score is ≥29, and anxiety-free if the score is <7 and the HAMD scores reaching up to 64. Higher scores indicate greater severity of depression. The degree of depression is considered severe if the score is ≥35, and depressionfree if the score is <8. Treatment effectiveness was categorized as; cure (complete restoration of all indicators to normal, disappearance of clinical symptoms, and no signs of relapse), improvement (improvement in all indicators, and improvement in clinical symptoms), and ineffective (no enhancement in clinical symptoms and occurrence of relapse). Calculation of the total effectiveness rate involved dividing the sum of cured and improvement cases by the total number of cases, and then multiplying by 100 %. The side effects like skin rashes, facial irritation, headaches, and nausea was recorded. To calculate the rate of adverse reactions, the number of patients was divided by the total number of cases, multiplied by 100 %. Utilizing the Statistical Package for the Social Sciences (SPSS) 25.0, the analysis was performed, comparing the measurement data, presented as mean±standard deviation. Utilizing Chi-square (χ2) test, the count data were analyzed. Patients in the observation group exhibited notably shorter duration, clinical symptom improvement compared to the control (p<0.05) as shown in fig. 1. Initially, no noteworthy variations in rosacea symptom scores were seen between two groups. Yet, subsequent to treatment, there was a considerable reduction in symptom scores for both groups, demonstrating lower scores than the control group as shown in Table 1. Following treatment, the HAMA scores and HAMD scores of patients in the observation were decreased compared to control, signifying a remarkable variation (p<0.05) as shown in Table 2. The overall treatment effectiveness rate in the observation group surpassed, demonstrating a noteworthy difference, described in Table 3. In the observation group, there were 4 cases of skin rash, facial irritation, headache, and nausea. Within the control group, adverse reactions were reported in 5 cases. The rate of adverse reactions in the observation was 8.89 %, lower than the 11.11 % in the control, as shown in Table 4. The cause of rosacea remains unknown, despite being a chronic inflammatory skin disorder[14]. It is estimated that over 5 % of the global population suffers from rosacea[15], and it can occur in all age groups, with the highest prevalence around the age of 30 y, with more incidence in women. Although the specific pathogenesis of rosacea remains incompletely elucidated, studies have suggested that various stimuli lead to upregulation of toll-like receptor 2 expression in keratinocytes, activating the inflammatory cascade mediated by the antimicrobial peptide LL37, recruiting various inflammatory cells, generating reactive oxygen species, causing damage to the skin’s natural defense and barrier functions, promoting angiogenesis and fibrosis, ultimately leading to the onset of rosacea[16]. Hydroxychloroquine has various effects, like anti-inflammatory, immunomodulatory, anti-proliferative, and protection against Ultra-Violet (UV) damage[17]. However, in clinical practice, it has been found that treating alone with hydroxychloroquine may lead to recurrence. Azithromycin belongs to the second generation of macrolide antibiotics, has good tissue penetration, and is rapidly absorbed and widely distributed in the body, with a half-life of up to 68 h. After discontinuation, it continues to inhibit bacteria, as recommended in the Chinese Rosacea Diagnosis and Treatment Guidelines (2021 edition)[18]. Additionally, it has been reported that compared to first-generation macrolides such as erythromycin, azithromycin has less organ metabolism toxicity, and higher safety[19]. This research investigated the clinical effectiveness of hydroxychloroquine in conjunction with azithromycin for treating rosacea through retrospective analysis. The findings revealed that the duration of clinical symptom improvement in the observation group was notably shorter than that in the control. Moreover, the observation group exhibited better rosacea symptom scores, HAMA scores, and HAMD scores following treatment. Additionally, it also exhibited a higher overall treatment effectiveness rate. Hydroxychloroquine may alleviate the disease through its anti-inflammatory and immunomodulatory effects, while azithromycin’s antibacterial and antiinflammatory properties may further enhance the treatment effect. The superior efficacy of the observation group may be attributed to the broader spectrum of action with the combined treatment regimen, which can intervene in the pathogenesis of the disease from different aspects. The variance did not attain statistical significance, suggesting that combination therapy did not substantially increase the risk of adverse reactions. Underlining the positive findings of this study, it’s important to be aware of its limitations. First of all, the study used a retrospective analysis design, which carries the potential risk of information retrieval and selection bias. Another consideration is the relatively small sample size, highlighting the need for larger-scale clinical studies to confirm the results. Furthermore, the study duration was limited, requiring an extended follow-up to assess long-term efficacy and safety. In summary, the combination therapy of hydroxychloroquine and azithromycin demonstrates significant advantages in ameliorating the clinical symptoms and psychological well-being of patients with rosacea. Although the results of this study have some clinical significance, further research is needed to verify its efficacy and safety, and to provide more accurate guidance for the treatment of rosacea. Finally, we hope that the results of our study can provide clinicians with more treatment options for rosacea, and serve as a reference for future clinical research.
| Group (n=45) | Symptom score | t | p | |
|---|---|---|---|---|
| Before | After | |||
| Observation | 2.31±0.47 | 0.89±0.32 | 16.860 | 0.000 |
| Control | 2.27±0.45 | 1.42±0.50 | 8.449 | 0.000 |
| t | -0.460 | -6.043 | ||
| p | 0.646 | 0.000 | ||
Table 1: Symptom Score (x±s, Points)
| Group (n=45) | HAMA | HAMD | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Observation | 44.13±4.94 | 10.18±2.39* | 47.20±4.41 | 11.71±2.40* |
| Control | 44.36±4.46 | 18.67±2.84* | 46.78±4.64 | 19.51±2.79* |
| t | 0.224 | 15.362 | -0.442 | 14.211 |
| p | 0.823 | 0.000 | 0.659 | 0.000 |
Note: (*) indicates noteworthy difference following treatment compared with prior to treatment
Table 2: HAMA Scores and HAMD Scores (x±s, Points)
| Group (n=45) | Cured | Improvement | Ineffectiveness | Overall effective rate |
|---|---|---|---|---|
| Observation | 23 (51.11) | 20 (44.44) | 2 (4.44) | 43 (95.56) |
| Control | 18 (40.00) | 17 (37.78) | 10 (22.22) | 35 (77.78) |
| χ2 | 6.154 | |||
| p | 0.013 |
Table 3: Curative Effect
| Group (n=45) | Rash | Facial stimulation | Headache | Nausea | Overall incidence |
|---|---|---|---|---|---|
| Observation | 1 (2.22) | 1 (2.22) | 1 (2.22) | 1 (2.22) | 4 (8.89) |
| Control | 1 (2.22) | 2 (4.44) | 0 (4.44) | 2 (4.44) | 5 (11.11) |
| χ2 | 0.123 | ||||
| p | 0.725 |
Table 4: Complications n (%)
Conflict of interests:
The authors declared no conflict of interests.
References
- Dai R, Lin B, Zhang X, Lou Y, Xu S. Depression and anxiety in rosacea patients: A systematic review and meta-analysis. Dermatol Ther 2021;11(6):2089-105.
[Crossref] [Google Scholar] [PubMed]
- Zeichner JA, Eichenfield LF, Feldman SR, Kasteler JS, Ferrusi IL. Quality of life in individuals with erythematotelangiectatic and papulopustular rosacea: Findings from a web-based survey. J Clin Aesthetic Dermatol 2018;11(2):47-52.
[Google Scholar] [PubMed]
- van Zuuren EJ, Arents BW, vander Linden MM, Vermeulen S, Fedorowicz Z, Tan J. Rosacea: New concepts in classification and treatment. Am J Clin Dermatol 2021;22(4):457-65.
[Crossref] [Google Scholar] [PubMed]
- Schaller M, Almeida LM, Bewley A, Cribier B, Dlova NC, Kautz G, et al. Rosacea treatment update: Recommendations from the global Rosacea Consensus (ROSCO) panel. Br J Dermatol 2017;176(2):465-71.
[Crossref] [Google Scholar] [PubMed]
- Tan J, Almeida LM, Bewley A, Cribier B, Dlova NC, Gallo R, et al. Updating the diagnosis, classification and assessment of rosacea: Recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol 2017;176(2):431-8.
[Crossref] [Google Scholar] [PubMed]
- Venetsanopoulou AI, Voulgari PV, Drosos AA. Advances in non-biological drugs for the treatment of rheumatoid arthritis. Exp Opin Pharmacother 2024;25(1):45-53.
[Crossref] [Google Scholar] [PubMed]
- Stoll F, Blank A, Mikus G, Czock D, Weiss J, Meyer-Tönnies MJ, et al. Evaluation of hydroxychloroquine as a perpetrator on cytochrome P450 (CYP) 3A and CYP2D6 activity with microdosed probe drugs in healthy volunteers. Eur J Drug Metab Pharmacokinet 2024;49(1):101-9.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Zhou S, Fan X, Wang Z. Effect of multifunctional laser photoelectricity platform combined with hydroxychloroquine treatment sensitive facial skin. Dermatol Ther 2022;35(11):e15795.
[Crossref] [Google Scholar] [PubMed]
- Barton VR, Toussi A, Awasthi S, Kiuru M. Treatment of pediatric alopecia areata: A systematic review. J Am Acad Dermatol 2022;86(6):1318-34.
[Crossref] [Google Scholar] [PubMed]
- Avraham S, Khaslavsky S, Kashetsky N, Starkey SY, Zaslavsky K, Lam JM, et al. Treatment of ocular rosacea: A systematic review. J Der Dtsch Dermatol Ges 2024;22(2):167-74.
[Crossref] [Google Scholar] [PubMed]
- Choe J, Barbieri JS. Emerging medical therapies in rosacea: A narrative review. Dermatol Ther 2023;13(12):2933-49.
[Crossref] [Google Scholar] [PubMed]
- Brémond-Gignac D, Navel V, Doan S, Chiambaretta F. Paediatric ocular rosacea: Diagnosis and management with an eyelid-warming device and topical azithromycin 1.5 %. J Fr Ophtalmol 2022;45(10):1150-9.
[Crossref] [Google Scholar] [PubMed]
- Gomolin T, Cline A, Pereira F. Treatment of rosacea during pregnancy. Dermatol Online J 2021;27(7).
[Crossref] [Google Scholar] [PubMed]
- Del Rosso J, Baldwin H, Bhatia N, Chavda R, York JP, Harper J, et al. A review of the diagnostic and therapeutic gaps in rosacea management: Consensus opinion. Dermatol Ther 2024;14(2):271-84.
[Crossref] [Google Scholar] [PubMed]
- Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: A systematic review and meta-analysis. Br J Dermatol 2018;179(2):282-9.
[Crossref] [Google Scholar] [PubMed]
- Moura AK, Guedes F, Rivitti-Machado MC, Sotto MN. Innate immunity in rosacea. Langerhans cells, plasmacytoid dentritic cells, Toll-like receptors and inducible oxide nitric synthase (iNOS) expression in skin specimens: Case-control study. Arch Dermatol Res 2018;310(2):139-46.
[Crossref] [Google Scholar] [PubMed]
- Li J, Yuan X, Tang Y, Wang B, Deng Z, Huang Y, et al. Hydroxychloroquine is a novel therapeutic approach for rosacea. Int Immunopharmacol 2020;79:106178.
[Crossref] [Google Scholar] [PubMed]
- Gu H, Hao F, He W, Jian D, Jian Z, Jiang X, et al. Guidelines for the diagnosis and treatment of rosacea in China (2021 Edition). Int J Dermatol Venereol 2021;4(4):199-209.
- Atieh MA, Shah M, Hakam A, Alghafri M, Tawse-Smith A, Alsabeeha NH. Systemic azithromycin vs. amoxicillin/metronidazole as an adjunct in the treatment of periodontitis: A systematic review and meta-analysis. Aust Dent J 2023;69(1):4-17.
[Crossref] [Google Scholar] [PubMed]