- *Corresponding Author:
- Chunyi Xia
Department of Neurosurgery, Shenyang First People’s Hospital of Liaoning Province, Shenyang, Liaoning Province 110041, P. R. China
E-mail: fuzhijian1975@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “342-346” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study seeks to examine the effectiveness and safety of lacosamide in treating pediatric individuals with focal seizures. 120 children diagnosed with either new-onset or refractory focal seizures involved in the study and were randomly allocated into either the control or observation group. Conventional treatment, comprising oral oxcarbazepine, was administered to the control group, whereas the observation group received lacosamide in addition to conventional treatment. Both sets of participants were administered medication for a period of 12 mo. A comparison was made in terms of seizure frequency, treatment effectiveness, intelligence quotient levels, quality of life ratings, and adverse reactions between groups. Prior to treatment, there was no notable contrast in seizure frequency between the groups (p>0.05). Nonetheless, following 6 and 12 mo of treatment, the observation group demonstrated notably decreased seizure frequency as opposed to the control group (p<0.05). The overall treatment effectiveness in the observation group stood considerably higher than that of the control group (p<0.05). Although no discernible disparities in intelligence quotient levels was observed prior to treatment (p>0.05), posttreatment, the intelligence quotient level was notably elevated in the observation group as opposed to the control group, with noteworthy improvement observed in both groups in relative to pre-treatment levels (p<0.05). No remarkable difference was found in the scores of physiological function, school function, emotional function, and social function prior to treatment (p>0.05). Subsequent to treatment, the observation group exhibited markedly elevated scores, and both groups revealed substantial progress from their scores before treatment (p<0.05). The occurrence of adverse reactions did not demonstrate noteworthy differences between groups (p>0.05). The combined administration of lacosamide and oxcarbazepine displayed superior effectiveness in managing focal seizures in pediatric patients compared to the use of oxcarbazepine alone. It caused a marked reduction in seizure frequency and had a favorable impact on the intellectual capacity and life quality of the patients. Lacosamide maintained a positive safety profile, with no noticeable increase in adverse reaction frequency.
Keywords
Lacosamide, pediatric epilepsy, focal seizures, efficacy, safety
Epilepsy in children is a frequent neurological ailment, yet its root causes remain inadequately elucidated. It appears to be predominantly related to variables such as cranial trauma, intracranial infections, cerebral oxygen deficiency, and atypical brain maturation[1,2]. Abrupt neuronal discharges within a patient’s brain can impair cognitive function. Prolonged and recurring epilepsy can substantially affect the well-being of afflicted children, place strain on families and society, and in severe instances, contribute to lasting brain impairment, influencing children’s physical and intellectual growth[3-5]. Consequently, timely intervention is pivotal in managing the ailment in pediatric patients.
Presently, the prevailing method for treating epilepsy involves medication therapy. Nonetheless, using a single drug often leads to subpar results and might eventually lead to drug-resistant epilepsy. Combining new medications or multiple drugs has demonstrated enhanced efficacy in managing focal seizures[6-8]. Numerous antiepileptic medications have been created and extensively integrated into clinical settings over the recent decades[9]. Despite this, managing focal seizures in pediatric patients remains challenging. Traditional antiepileptic drugs might exhibit limited effectiveness and could lead to severe adverse responses and longlasting cognitive impacts.
Lacosamide, a modern antiepileptic agent, modulates neuronal electrical activity to hinder aberrant neuronal discharges[10-12]. It has exhibited favorable effectiveness and a comparatively low frequency of adverse effects in adult patients with epilepsy[13,14]. Notwithstanding, further exploration is necessary to evaluate the effectiveness and safety of lacosamide in treating focal seizures in pediatric patients.
This research seeks to evaluate the effectiveness and safety of lacosamide in managing focal seizures in pediatric individuals. Through systematic monitoring and continued observation of the patient cohort, we aim to evaluate the effects of lacosamide on seizure frequency, severity, and any potential adverse reactions and side effects. The outcomes of this study will supply clinicians with further knowledge to assist in the utilization of lacosamide in pediatric patients, contributing to an enhanced quality of life for children struggling with epilepsy.
Materials and Methods
General information:
New-onset or refractory focal seizures, all of whom were attended to our pediatric outpatient and inpatient units from January 2021 to January 2023. Based on varied treatment modalities, the individuals were segregated into the control and observation groups. Of the individuals in the control group, 36 were male, and 24 were female, aged between 4 y and 15 y (9.87±2.36) y. The disease duration in this cohort ranged from 8 mo to 7 y (4.15±1.12) y, and the weight of the participants ranged from 12 to 50 (30.39±8.96) kg. Among these individuals, 40 were newly diagnosed with focal seizures, while 20 had refractory focal seizures. Within the observation group, there were 38 males and 22 females, aged 4-16 (10.12±2.63) y. The disease duration in this group ranged from 6 mo to 8 y (4.16±1.04) y. The weight of the participants ranged from 11 to 48 (29.74±8.77) kg. 35 participants were newly diagnosed with focal seizures and 25 had refractory focal seizures. No remarkable variations in general information were observed between groups (p>0.05), suggesting their comparability. Approval for this research was granted by the hospital’s ethics committee, and the parents of the patients were educated about the study’s intent and significance. They voluntarily agreed to participate in the treatment and follow-up, and provided written informed consent.
Inclusion criteria: Age range of 4 y-16 y and clinically diagnosed and confirmed as new-onset or refractory focal seizures[10].
Exclusion criteria: Allergy to lacosamide or severe intolerance; existence of 2nd or 3rd degree atrioventricular block; severe liver or kidney dysfunction and partially incomplete clinical data. The patients were then segregated into the control and treatment groups based on different treatment methods, with 30 cases in each group.
Methods:
Standard treatment was administered to the control group, involving the oral administration of oxcarbazepine (Manufacturer: Novartis Pharma Schweiz AG, Registration No: H20130015, Specifications: 0.15 g) at an initial dosage of 10 mg/ (kg·d). This was incremented by 5 mg/(kg·d) every 2 w, and later maintained at 20 mg/(kg·d). As a supplement to the standard treatment, the observation group received lacosamide. They were administered lacosamide (Manufacturer: Aesica Pharmaceuticals GmbH, Registration No: H20180063, Specifications: 100 mg/tablet×14 tablets). Individuals weighing from 11-30 kg initiated treatment with a starting dose of 2 mg/(kg·d), and it was progressively raised by 2 mg/ (kg·d) each week, ultimately reaching a maintenance dose of 4-8 mg/(kg·d). For those weighing over 30 kg up to 50 kg, the initial dose was 2 mg/(kg·d), and it was similarly raised by 2 mg/(kg·d) each week, culminating in a maintenance dose between 6-12 mg/(kg·d). Both sets of patients followed the medication regimen for duration of 12 mo, and the patient’s families received intensive health education throughout this period. This educational program focused on illuminating the underlying causes of focal seizures, available treatment modalities, expected outcomes, and practical aspects of daily living. Emphasis was placed on the significance of medication treatment and the possible adverse effects to bolster the families’ comprehension and collaboration with the treatment.
Observational indicators and determination criteria:
Comparison of seizure frequency: Seizure frequencies for the patients were recorded by their parents prior to treatment and at 6 mo and 12 mo of treatment.
Comparison of treatment efficacy: Clinical efficacy was defined based on the percentage improvement in seizure frequency. Patients were followed up regularly after 6 mo of treatment, and the follow-up was completed at 12 mo. Patients without seizures during this period were rated as having no seizures. A reduction in seizure frequency of ≥50 % was considered effective, while a reduction of <50 % was deemed ineffective. Total effectiveness=no seizures+effective.
Comparison of Intelligence Quotient (IQ) levels prior to and following treatment: The Wechsler Preschool and Primary Scale of Intelligence (WPPSI) was administered for children aged (4-6) y, while the Wechsler Intelligence Scale for Children (WISC) was utilized for those aged (7-16) y. The average IQ score was 100, with a standard deviation of 15, showing a moderately broad effective range of 55-145. The conversion method was IQ=100+(X-M)/SD×15.
Comparison of life quality scores prior to and following treatment: An in-house questionnaire was employed to evaluate variations in the children’s quality of life prior to and following treatment. The questionnaire encompassed four aspects; physical function, academic function, emotional well-being, and social interaction, with scoring from 0-100. A greater score denoted an improved quality of life. Comparison of the occurrence of adverse reactions, including dizziness, headache, drowsiness, nausea, vomiting, blurred vision and diplopia during medication.
Statistical analysis:
The analysis compared the measurement data between the two groups utilizing the Statistical Package for the Social Sciences (SPSS) 25.0 software, presented as mean±standard deviation, and employed the Chisquare (χ2) test for analyzing the count data, with notable significance defined at p<0.05.
Results and Discussion
Prior to the commencement of treatment, no notable disparities in seizure frequency were identified between groups (p>0.05). Nevertheless, at the 6 mo and 12 mo marks of the treatment period, the observation group exhibited a remarkably reduced seizure frequency as opposed to the control group, and this variation was noteworthy (p<0.05) as shown in Table 1.
| Group (n=60) | Pre-treatment | 6 mo after treatment | 12 mo after treatment |
|---|---|---|---|
| Observation | 15.63±2.71 | 6.12±1.43* | 3.22±0.58*# |
| Control | 14.88±2.31 | 7.65±1.38* | 4.73±1.18*# |
| t | -1.631 | 5.992 | 8.939 |
| p | 0.105 | 0.000 | 0.000 |
Note: *p<0.005 and #p<0.001
Table 1: Seizure Frequency
The overall effectiveness in the observation group indicated a substantial elevation as opposed to the control group, with substantial significance (p<0.05) as shown in Table 2.
| Group (n=60) | Absence of seizure | Effective | Ineffective | Overall effective rate |
|---|---|---|---|---|
| Observation | 20 (33.33) | 29 (48.33) | 13 (21.67) | 47 (78.33) |
| Control | 16 (26.67) | 23 (38.33) | 21 (35.00) | 39 (65.00) |
| χ2 | 4.261 | |||
| p | 0.039 | |||
Table 2: Curative Effect
No remarkable differences in IQ levels between groups was observed prior to treatment (p>0.05). However, following treatment, the IQ levels in both groups rose compared to before treatment, with the observation group demonstrating a notably higher level (p<0.05) as shown in fig. 1.
The scores for physical, academic, emotional, and social functioning did not remarkably differ between groups prior to treatment (p>0.05). Nevertheless, following treatment, the scores in physiological function, academic function, emotional function, and social function all saw an increase, and the observation group attained substantially higher scores than the control group (p<0.05) (Table 3). Both groups displayed a similar incidence of adverse reactions, and the variance was not markedly significant (p>0.05) as shown in Table 4.
| Group (n=60) | Physiological function | School function | Affective function | Social function | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Observation | 53.18±6.14 | 73.33±9.53 | 57.58±6.06 | 76.75±8.48 | 54.93±6.74 | 68.13±7.38 | 54.88±5.22 | 77.07±7.05 |
| Control | 54.67±6.39 | 68.35±8.65 | 56.93±6.79 | 70.90±8.44 | 55.72±6.45 | 61.57±6.83 | 54.52±6.17 | 68.12±7.04 |
| t | 1.297 | -3.000 | -0.553 | -3.788 | 0.651 | -5.058 | -0.352 | -6.954 |
| p | 0.197 | 0.003 | 0.581 | 0.000 | 0.516 | 0.000 | 0.726 | 0.000 |
Table 3: Periodontal Index (X±S, Points)
| Group (n=60) | Dizziness and headache | Nausea and vomiting | Drowsiness | Blurred vision/double vision | Overall incidence |
|---|---|---|---|---|---|
| Observation | 1 (1.67) | 2 (3.33) | 1 (1.67) | 2 (3.33) | 6 (10.00) |
| Control | 2 (3.33) | 1 (1.67) | 3 (5.00) | 1 (1.67) | 7 (11.67) |
| χ2 | 0.086 | ||||
| p | 0.769 | ||||
Table 4: Adverse Reactions N (%)
At present, the predominant mode of addressing pediatric epilepsy is centered around the administration of antiepileptic drugs[15,16]. Commonly employed medications for children encompass oxcarbazepine, valproic acid, lamotrigine, and levetiracetam[17,18].
Yet, a noteworthy portion of children suffering from epilepsy do not achieve adequate seizure management, highlighting the demand for innovative antiepileptic drugs with improved effectiveness, tolerance, and/or pharmacokinetic characteristics. Our study highlighted the impact of lacosamide, an amino acid derivative, as a novel antiepileptic compound that manages the gradual deactivation of sodium ion channels and alters neuronal signal conduction mediated by the Collapsin Response Mediator Protein-2 (CRMP-2) protein[19]. Notably, our findings revealed the substantial benefits of combining lacosamide with oxcarbazepine over the use of oxcarbazepine alone, demonstrating improved reduction in seizure frequency and treatment efficacy in pediatric patients affected by focal seizures. Upon comparison between the observation and control groups, it was evident that the observation group exhibited notably reduced seizure frequencies in contrast to the control group with a duration of 6 and 12 mo, correlating to a higher comprehensive efficacy rate. This underscores the favorable efficacy of lacosamide in managing seizure episodes.
Moreover, we witnessed progress in the IQ levels and quality of life among both groups of children following treatment, with a more pronounced increase in the IQ levels noted in the observation group compared to the control group. This indicates a beneficial impact of lacosamide on the cognitive development of children. Moreover, the observation group showcased considerably elevated scores in physiological function, school function, emotional function, and social function compared to their pre-treatment levels, as well as in contrast to the control group. This reinforces the role of lacosamide treatment in enhancing the children’s daily life and social functions. An investigation into the long-term safety, tolerability, and effectiveness of adding lacosamide in managing Chinese and Japanese individuals with epilepsy, conducted as a phase III, double-blind, open-label randomized trial, revealed good tolerability of lacosamide during extended treatment, without any notable escalation in the occurrence of seizures after 36 mo of therapy[20], consistent with the findings of our study.
In the context of safety, there were no substantial discrepancies in the frequency of adverse reactions between groups. Lacosamide was well-tolerated, further affirming its potential as an alternative therapeutic option for focal seizures. However, we recognize certain constraints in our study, including the relatively small sample size and constrained observation duration. More extensive randomized controlled trials are imperative to corroborate our findings and to conduct a more extensive appraisal of the long-term effectiveness and safety of lacosamide in children with focal seizures.
To sum up, our study indicates that the concurrent utilization of lacosamide and oxcarbazepine offers an effective, safe treatment approach, with the potential to enhance results in children with focal seizures. This combined therapy markedly reduces seizure frequency and positively influences cognitive development and overall quality of life in children.
Author’s contributions:
Zhijian Fu and Di Sun have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cepeda C, Wu J. Basic mechanisms of pediatric epilepsy. CNS Neurosci Ther 2015;21(2):71.
[Crossref] [Google Scholar] [PubMed]
- Striano P, Minassian BA. From genetic testing to precision medicine in epilepsy. Neurotherapeutics 2020;17(2):609-15.
[Crossref] [Google Scholar] [PubMed]
- Symonds JD, Elliott KS, Shetty J, Armstrong M, Brunklaus A, Cutcutache I, et al. Early childhood epilepsies: Epidemiology, classification, aetiology, and socio-economic determinants. Brain 2021;144(9):2879-91.
[Crossref] [Google Scholar] [PubMed]
- Symonds JD, Zuberi SM, Stewart K, McLellan A, O ‘Regan M, MacLeod S, et al. Incidence and phenotypes of childhood-onset genetic epilepsies: A prospective population-based national cohort. Brain 2019;142(8):2303-18.
[Crossref] [Google Scholar] [PubMed]
- Urio OH, Kija E, Weckhuysen S, Makungu H, Naburi H. Drug resistant epilepsy and associated factors among children with epilepsies in tanzania: A cross-sectional study. BMC Neurol 2024;24(1):8.
[Crossref] [Google Scholar] [PubMed]
- Moosa AN. Antiepileptic drug treatment of epilepsy in children. Continum 2019;25(2):381-407.
[Google Scholar] [PubMed]
- Dichter MA, Brodie MJ. New antiepileptic drugs. New Engl J Med 1996;334(24):1583-90.
- Chen Z, Brodie MJ, Kwan P. What has been the impact of new drug treatments on epilepsy? Curr Opin Neurol 2020;33(2):185-90.
[Crossref] [Google Scholar] [PubMed]
- Abou-Khalil BW. Update on antiepileptic drugs 2019. Continuum 2019;25(2):508-36.
[Google Scholar] [PubMed]
- Yang C, Yang Y, Peng Y, Zhang L, Yu D. Efficacy and safety of lacosamide in pediatric patients with epilepsy: A systematic review and meta-analysis. Epilepsy Behav 2022;134:108781.
[Crossref] [Google Scholar] [PubMed]
- Carona A, Bicker J, Silva R, Fonseca C, Falcao A, Fortuna A. Pharmacology of lacosamide: From its molecular mechanisms and pharmacokinetics to future therapeutic applications. Life Sci 2021;275:119342.
[Crossref] [Google Scholar] [PubMed]
- Babar RK, Bresnahan R, Gillespie CS, Michael BD. Lacosamide add-on therapy for focal epilepsy. Cochrane Database Syst Rev 2021;5(5):Cd008841.
[Crossref] [Google Scholar] [PubMed]
- Rudd GD, Haverkamp W, Mason JW, Wenger T, Jay G, Hebert D, et al. Lacosamide cardiac safety: Clinical trials in patients with partial-onset seizures. Acta Neurol Scand 2015;132(5):355-63.
[Crossref] [Google Scholar] [PubMed]
- Li J, Sun M, Wang X. The adverse-effect profile of lacosamide. Expert Opin Drug Saf 2020;19(2):131-8.
[Crossref] [Google Scholar] [PubMed]
- Kopciuch D, Kus K, Fliciński J, Steinborn B, Winczewska-Wiktor A, Paczkowska A, et al. Pharmacovigilance in pediatric patients with epilepsy using antiepileptic drugs. Int J Environ Res Public Health 2022;19(8):4509.
[Crossref] [Google Scholar] [PubMed]
- Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust 2018;208(5):226-33.
[Crossref] [Google Scholar] [PubMed]
- Abou-Khalil BW. Antiepileptic drugs. Continuum (Minneap Minn) 2016;22(1):132-56.
- Zelleke T, Pasupuleti A, Depositario-Cabacar D. Antiepileptic drugs in pediatrics. Handb Exp Pharmacol 2020;261:1-24.
- Rogawski MA, Tofighy A, White HS, Matagne A, Wolff C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res 2015;110:189-205.
[Crossref] [Google Scholar] [PubMed]
- Inoue Y, Liao W, Wang X, Du X, Tennigkeit F, Sasamoto H, et al. Safety and efficacy of adjunctive lacosamide in Chinese and Japanese adults with epilepsy and focal seizures: A long-term, open-label extension of a randomized, controlled trial. Epilepsy Res 2021;176:106705.
[Crossref] [Google Scholar] [PubMed]
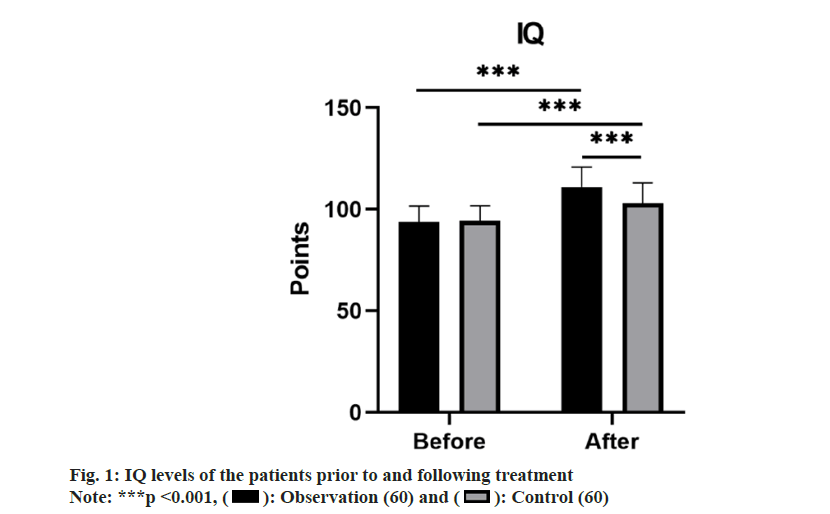
 ): Observation (60) and (
): Observation (60) and ( ): Control (60)
): Control (60)



