- *Corresponding Author:
- Xue Bai
Department of Neurology,
The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University,
Luzhou, Sichuan 646000,
China
E-mail: baixue@swmu.edu.cn
| This article was originally published in a special issue,“New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “180-190” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Based on the theory of “Brain Xuan-fu Blood-Brain Barrier”, we use network pharmacology to study the potential mechanism of wind medicine to regulate the blood-brain barrier. The effective components of six wind medicines were screened using the traditional Chinese medicine systems pharmacology database and analysis platform and relevant targets were identified through this database; potential targets related to blood-brain barrier function were obtained through the GeneCards database; the “drug-component-target-blood-brain barrier” network map was constructed using Cytoscape software. The search tool for the retrieval of interacting genes/proteins database was used to construct a protein-protein interaction network map of target protein interactions and to screen the core targets using Cytoscape software; the database for annotation, visualization and integrated discovery database was used to perform gene ontology and Kyoto encyclopedia of genes and genomes enrichment analysis of the core targets at the drug-disease intersection; Vina was used for molecular docking. Six wind drugs with 43 effective components and 255 potential targets were screened, with a total of 217 targets where the drug intersected with the blood-brain barrier and 68 core targets. The top 20 biological processes obtained were related to pathways such as positive regulation of ribonucleic acid polymerase II promoter transcription, positive regulation of transcription, negative regulation of the apoptotic process, inflammatory response, lipopolysaccharide-mediated signaling pathway and cellular response to hypoxia. The top 20 pathways obtained involved cancer pathways, tumor necrosis factor signaling pathway, T cell receptor signaling pathway, nucleotide-binding oligomerization domain-like receptor signaling pathway, hypoxia-inducible factor 1 signaling pathway, toll-like receptor signaling pathway, etc. The molecular docking results showed that the main effective components bind well to the core targets. The mechanism of action of wind medicine is probably through the mechanism of anti-inflammation and anti-apoptosis to act on the blood-brain barrier, which is of great significance in the treatment of encephalopathy.
Keywords
Wind medicine, blood-brain barrier, network pharmacology, encephalopathy
The Blood-Brain Barrier (BBB) maintains the homeostasis of the brain’s internal environment and protects the central nervous system from toxins, pathogens, inflammation, etc[1]. When brain diseases occur, its barrier effect is disrupted, on the one hand, the increased permeability will allow harmful substances to enter the central nervous system, etc., and on the other hand, its barrier function will create barriers to drug delivery to the central nervous system[2]. Studies have confirmed that BBB disruption is an important factor and pathological marker in the development of stroke, vascular dementia, Alzheimer’s disease and other central nervous system diseases[3,4]. Therefore,it is important to study the regulation of BBB function for the treatment of central nervous system diseases. The brain Xuan-fu is the manifestation of the Xuan-fu theory in the brain and has been widely used in recent years in the study of stroke, dementia and other brain diseases. Studies have shown that there is a significant correlation between the Xuan-fu of the brain and the BBB in terms of structure, physiological function and pathological characteristics[5]. The main pathogenesis of encephalopathy is the depression of the brain Xuan-fu and the treatment should be to open the Xuan-fu. Professor Mingjie Wang[6] believed that wind medicine is ascending and dispersing in nature, which is good at ascending and dispersing to the surface, can dispel and smooth the qi of the whole body and can draw medicine into the brain, so the use of wind medicine to open the Xuan-fu is a major rule in the treatment of encephalopathy. Studies have proved that the use of wind medicine has a significant regulatory effect on the function of BBB, which can reduce pathological BBB permeability and improve BBB function[7,8].
In this paper, we used the network pharmacology method to screen the effective components of selected wind medicine, predict their potential targets and reveal the network relationship between “wind drugcomponent- target-BBB” to explore the mechanism of action of wind drugs on the BBB.
Materials and Methods
Materials:
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://tcmspw.com/tcmsp.php); Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org/); Database for Annotation, Visualization and Integrated Discovery (DAVID) database (https://david. ncifcrf.gov/tools.jsp). GeneCards database (https:// www.genecards.org/); Uniprot database (www. uniprot.org/); Cytoscape software (Version3.7.0); Microbiology Letter online mapping website (http:// www. bioinformatics.com.cn).
Wind medicine screening:
Six wind medicines were selected based on “New knowledge of wind medicine” by Professor Mingjie Wang[9], namely Chuanxiong rhizoma, Ephedrae herba, Bupleuri radix, Asari Radix et Rhizoma, Puerariae lobatae radix, Cinnamomi ramulus.
Screening of effective components and their potential targets:
All effective components of the six wind medicines were retrieved using the TCMSP platform and the effective components were screened by Oral Bioavailability (OB)≥30 %; Drug-Like properties (DL)≥0.18; crossing BBB coefficient≥-3 and Half- Life (HL)≥4. The potential targets of each effective components were then derived from this database and the obtained target protein names were normalized to human gene names in the Uniprot database.
Acquisition of BBB related targets and screening of drug intersection targets with the BBB:
The targets related to the BBB were obtained from the GeneCards database using the keyword "Bloodbrain barrier". The targets related to the effective components that have been normalized to human gene names in the Uniprot database were intersected with the targets related to the BBB to find the targets of the effective components of wind medicine acting on the BBB.
Drug-component-target-BBB network:
The data from screening of effective components and their potential targets and acquisition of BBB related targets and screening of drug intersection targets with the BBB were compiled and uploaded to Cytoscape 3.7.0 software to construct a “drug-componenttarget- BBB” network map for visualization and reconciliation.
Drug-BBB, Protein-Protein Interaction (PPI) network construction and screening of core targets:
The intersection targets of wind medicine effective components and the BBB were entered into the STRING database, and the species was defined as “Homo sapiens” to construct the PPI network of wind medicine action on the BBB. The obtained STRING files in Tab-Separated Values (TSV) format were uploaded to Cytoscape 3.7.0 software for network topology analysis and the core targets were filtered out with the condition that they were larger than the average value of degree.
Functional enrichment and biological pathway analysis:
Enter the core targets into the DAVID database, select "OFFICE_GENE_SYMBOL", "gene list" and select "Homo sapiens" for the species. After data submission, Gene Ontology (GO) bioprocess analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed. The top 20 biological processes and KEGG pathways with the smallest p-value were selected with p0.05 as the screening criterion.
Molecular docking:
The top 10 core targets were selected by degree value for molecular docking with the key effective components and the Protein Data Bank (PDB) format files of the targets were obtained in the PDB database (https://www.rcsb.org/), and then the water molecules and small molecule ligands of the target proteins were removed by Pymol software and saved as PDB format files. Download the Two Dimensional (2D) structure file of the effective components from the PubChem database (https://pubchem.ncbi.nlm. nih.gov/), save it in mol2 format and convert it to a Three-Dimensional (3D) structure mol2 format file using Chem3D software. The PDB format receptor files and mol2 format ligand files were converted into PDBQT format using AutoDock Tools 1.5.6 software, and then molecular docking was performed using Vina.
Results and Discussion
After removing duplicate data, a total of 43 effective components were screened for 6 wind medicines. The effective components were numbered according to which drug they belonged to, for example, (-)-taxifolin belonged to Cinnamomi ramulus and was numbered GZ1, Peroxyergosterol also belonged to Cinnamomi ramulus and was numbered GZ2. The components belonging to Chuanxiong rhizoma started with CX; those belonging to Ephedrae herba started with MH, those belonging to Bupleuri radix started with CH; those belonging to Asari Radix et Rhizoma started with XX and those belonging to Puerariae lobatae radix started with GG. The effective components are common to several drugs and are numbered with capital letters plus Arabic numerals, for example, Mandenol is a common component of Chuanxiong rhizoma and Ephedrae herba and is numbered A1 (Table 1).
| Mol ID | Molecule name | Number | OB (%) | BBB | DL | HL |
|---|---|---|---|---|---|---|
| MOL001736 | (-)-taxifolin | GZ1 | 60.51 | -1.02 | 0.27 | 14.37 |
| MOL011169 | Peroxyergosterol | GZ2 | 44.39 | 0.43 | 0.82 | 4.06 |
| MOL000392 | Formononetin | GG1 | 69.67 | 0.02 | 0.21 | 17.04 |
| MOL002959 | 3'-Methoxydaidzein | GG2 | 48.57 | -0.32 | 0.24 | 17.04 |
| MOL002135 | Myricanone | CX1 | 40.6 | -0.08 | 0.51 | 4.39 |
| MOL002140 | Perlolyrine | CX2 | 65.95 | 0.15 | 0.27 | 12.62 |
| MOL002157 | Wallichilide | CX3 | 42.31 | 0.73 | 0.71 | 6.85 |
| MOL000433 | Folsaeure | CX4 | 68.96 | -2.59 | 0.71 | 213.28 |
| MOL001645 | Linoleyl acetate | CH1 | 42.1 | 1.08 | 0.2 | 7.48 |
| MOL002776 | Baicalin | CH2 | 40.12 | -1.74 | 0.75 | 17.36 |
| MOL000354 | Isorhamnetin | CH3 | 49.6 | -0.54 | 0.31 | 14.34 |
| MOL004598 | 3,5,6,7-tetramethoxy-2-(3,4,5-trimethoxyphenyl)chromone | CH4 | 31.97 | 0.08 | 0.59 | 15.54 |
| MOL004609 | Areapillin | CH5 | 48.96 | -0.29 | 0.41 | 16.52 |
| MOL013187 | Cubebin | CH6 | 57.13 | -0.41 | 0.64 | 12.4 |
| MOL004718 | α-spinasterol | CH7 | 42.98 | 0.79 | 0.76 | 6.46 |
| MOL004628 | Octalupine | CH8 | 47.82 | 0.3 | 0.28 | 4.17 |
| MOL004644 | Sainfuran | CH9 | 79.91 | 0.23 | 0.23 | 8.58 |
| MOL004648 | Troxerutin | CH10 | 31.6 | -0.38 | 0.28 | 4.36 |
| MOL004702 | Saikosaponin c_qt | CH11 | 30.5 | -0.85 | 0.63 | 6.12 |
| MOL002823 | Herbacetin | MH1 | 36.07 | -0.65 | 0.27 | 14.8 |
| MOL000006 | Luteolin | MH2 | 36.16 | -0.84 | 0.25 | 15.94 |
| MOL001755 | 24-Ethylcholest-4-en-3-one | MH3 | 36.08 | 1.22 | 0.76 | 5.49 |
| MOL001771 | Poriferast-5-en-3beta-ol | MH4 | 36.91 | 1.14 | 0.75 | 5.07 |
| MOL002881 | Diosmetin | MH5 | 31.14 | -0.66 | 0.27 | 16.34 |
| MOL004328 | Naringenin | MH6 | 59.29 | -0.37 | 0.21 | 16.98 |
| MOL005043 | Campest-5-en-3beta-ol | MH7 | 37.58 | 0.94 | 0.71 | 4.43 |
| MOL005190 | Eriodictyol | MH8 | 71.79 | -0.54 | 0.24 | 15.81 |
| MOL005573 | Genkwanin | MH9 | 37.13 | -0.24 | 0.24 | 16.1 |
| MOL005842 | Pectolinarigenin | MH10 | 41.17 | -0.09 | 0.3 | 16.56 |
| MOL011319 | Truflex OBP | MH11 | 43.74 | 0.6 | 0.24 | 4.9 |
| MOL012140 | 4,9-dimethoxy-1-vinyl-b-carboline | XX1 | 65.3 | 0.72 | 0.19 | 9.85 |
| MOL012141 | Caribine | XX2 | 37.06 | -0.15 | 0.83 | 10.44 |
| MOL001460 | Cryptopin | XX3 | 78.74 | 0.41 | 0.72 | 21.25 |
| MOL001558 | Sesamin | XX4 | 56.55 | -0.08 | 0.83 | 13.44 |
| MOL002962 | (3S)-7-hydroxy-3-(2,3,4-trimethoxyphenyl)chroman-4-one | XX5 | 48.23 | 0.01 | 0.33 | 16.64 |
| MOL009849 | ZINC05223929 | XX6 | 31.75 | 0.12 | 0.83 | 14.12 |
| MOL001494 | Mandenol | A1 | 42 | 1.14 | 0.19 | 5.39 |
| MOL000359 | Sitosterol | A2 | 36.91 | 0.87 | 0.75 | 5.37 |
| MOL000358 | b-sitosterol | B1 | 36.91 | 0.99 | 0.75 | 5.36 |
| MOL004576 | Taxifolin | C1 | 57.84 | -0.8 | 0.27 | 14.41 |
| MOL000449 | Stigmasterol | D1 | 43.83 | 1 | 0.76 | 5.57 |
| MOL000422 | Kaempferol | D2 | 41.88 | -0.55 | 0.24 | 14.74 |
| MOL000098 | Quercetin | D3 | 46.43 | -0.77 | 0.28 | 14.4 |
Table 1: The Active Components of Chuanxiong, Ephedra, Pueraria Lobata, Guizhi, Bupleurum, Asarum
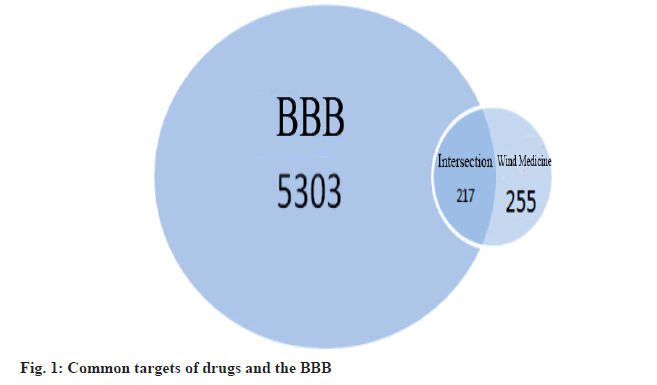
The relevant targets of 43 effective components were obtained from the TCMSP database, duplicate data were removed and entered into the Uniprot database normalized to human gene names, and targets for which the exact human gene names were not queried were removed. A total of 255 relevant targets were obtained. The 5303 BBB targets were obtained from the Genecards database and 217 intersecting targets were obtained by intersecting the wind drug-related targets with the BBB-related targets (fig. 1), including Interleukin-4 (IL-4), Tumour Necrosis Factor (TNF), Caspase-3 (CASP3), Mitogen-Activated Protein Kinase (MAPK) 8, etc.
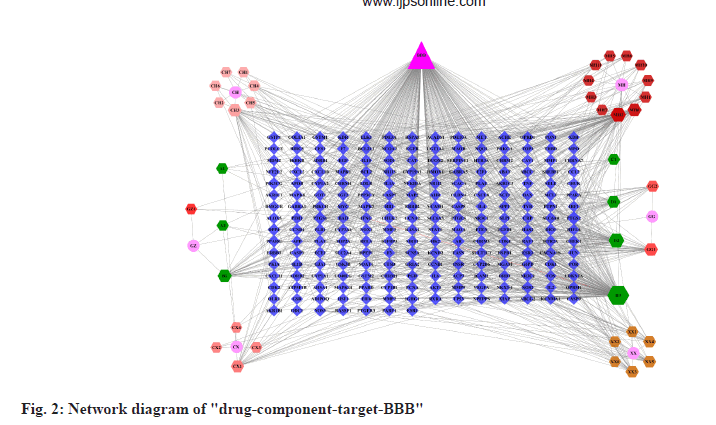
The data of the six wind medicines, the effective components (excluding those without relevant targets), the BBB and the intersection targets were input into Cytoscape 3.7.0 software for visualization, and the "drug-component-target-BBB" network diagram was obtained (fig. 2). The triangle represents the BBB, the hexagon represents the effective components, the circle represents the wind medicine and the diamond represents the intersection target (the color is to distinguish each part, no practical significance). CH represents Bupleuri radix, XX represents Asari Radix et Rhizoma, GG represents Puerariae lobatae radix, GZ represents Cinnamomi ramulus, CX represents Chuanxiong rhizoma and MH represents Ephedrae herba (Table 1). The node size and transparency are arranged according to degree values, the larger the degree value, the darker the color and the larger the node, the larger the degree value indicate the more important the node. From fig. 1, it can be seen that there are multiple compounds and multiple targets in six wind medicines, reflecting the existence of multiple components and multiple targets of wind medicines acting on the BBB, and it can be found that the first seven major components of the six wind medicines acting on the BBB are quercetin,kaempferol, beta (β)-sitosterol, isorhamnetin, luteolin and formononetin (Table 2).
| Serial number | Number | Components | Degree |
|---|---|---|---|
| 1 | D3 | Quercetin | 138 |
| 2 | D2 | Kaempferol | 54 |
| 3 | MH2 | Luteolin | 53 |
| 4 | MH6 | Naringenin | 34 |
| 5 | B1 | Beta-sitosterol | 33 |
| 6 | GG1 | Formononetin | 30 |
| 7 | CH3 | Isorhamnetin | 30 |
Table 2: Main Components of Wind Medicine Acting on the BBB
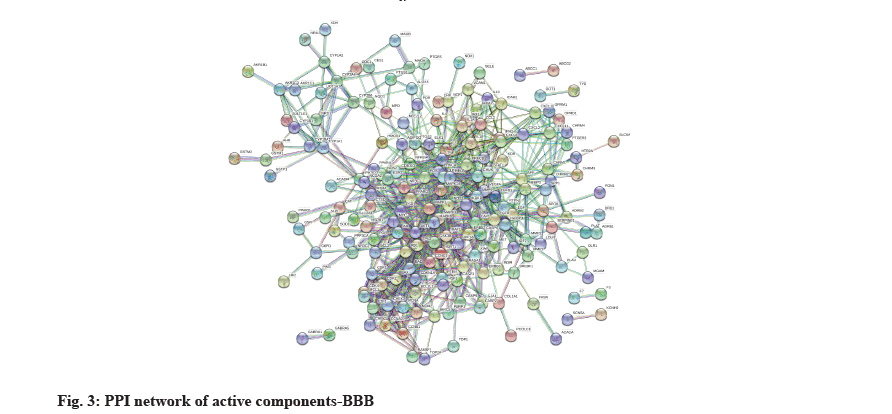
PPI network diagram of drug-target and core targets was described below. The 217 intersecting targets were imported into the STRING database (among which Immunoglobulin Heavy Constant Gamma 1 (IGHG1) was not recognized by the database), the species was limited to "Homo sapiens", the medium confidence was set >0.9 and the free targets were hidden to obtain the PPI network graph (fig. 3). There are 216 nodes (including the hidden free targets) and 859 edges, the nodes represent the target proteins and the edges represent the proteinprotein interactions, the average node degree is 7.95 and the average local clustering coefficient is 0.47. The obtained STRING file in TSV format was uploaded to Cytoscape 3.7.0 software for network topology analysis. The average value of degree was 9.187 and 68 core targets were screened with degree greater than 9.187 (Table 3).
| Number | Target | Degree | Number | Target | Degree |
|---|---|---|---|---|---|
| 1 | AKT1 | 42 | 35 | MDM2 | 14 |
| 2 | TP53 | 40 | 36 | AR | 14 |
| 3 | JUN | 38 | 37 | CYP1A1 | 14 |
| 4 | MAPK1 | 38 | 38 | SIRT1 | 14 |
| 5 | MAPK3 | 38 | 39 | CCL2 | 14 |
| 6 | TNF | 35 | 40 | TGFB1 | 14 |
| 7 | RELA | 34 | 41 | CXCL2 | 14 |
| 8 | MAPK8 | 28 | 42 | CHRM2 | 14 |
| 9 | IL-6 | 28 | 43 | BCL2L1 | 13 |
| 10 | MAPK14 | 27 | 44 | CCNA2 | 13 |
| 11 | APP | 26 | 45 | NCOA2 | 13 |
| 12 | EGFR | 25 | 46 | BCL2 | 13 |
| 13 | FOS | 23 | 47 | CXCL10 | 13 |
| 14 | MYC | 23 | 48 | IFNG | 13 |
| 15 | CXCL8 | 23 | 49 | PPARA | 13 |
| 16 | ESR1 | 23 | 50 | CCNB1 | 12 |
| 17 | RB1 | 22 | 51 | PCNA | 12 |
| 18 | VEGFA | 21 | 52 | PPARG | 12 |
| 19 | RXRA | 21 | 53 | IL-10 | 12 |
| 20 | CCND1 | 20 | 54 | APOB | 12 |
| 21 | NR3C1 | 20 | 55 | CYP3A4 | 12 |
| 22 | EGF | 19 | 56 | CDK4 | 11 |
| 23 | PRKCA | 18 | 57 | XIAP | 11 |
| 24 | CASP8 | 17 | 58 | E2F1 | 11 |
| 25 | CASP3 | 17 | 59 | GSK3B | 11 |
| 26 | CDKN1A | 17 | 60 | HIF1A | 11 |
| 27 | IL-1b | 17 | 61 | ERBB2 | 11 |
| 28 | PRKCB | 17 | 62 | CHUK | 10 |
| 29 | CDK1 | 16 | 63 | IGF2 | 10 |
| 30 | IL-4 | 16 | 64 | MMP9 | 10 |
| 31 | STAT1 | 16 | 65 | CXCL11 | 10 |
| 32 | CDK2 | 15 | 66 | PTGS2 | 10 |
| 33 | NFKBIA | 15 | 67 | NOS2 | 10 |
| 34 | IL-2 | 15 | 68 | RASA1 | 10 |
Table 3: Core targets of Drug-BBB
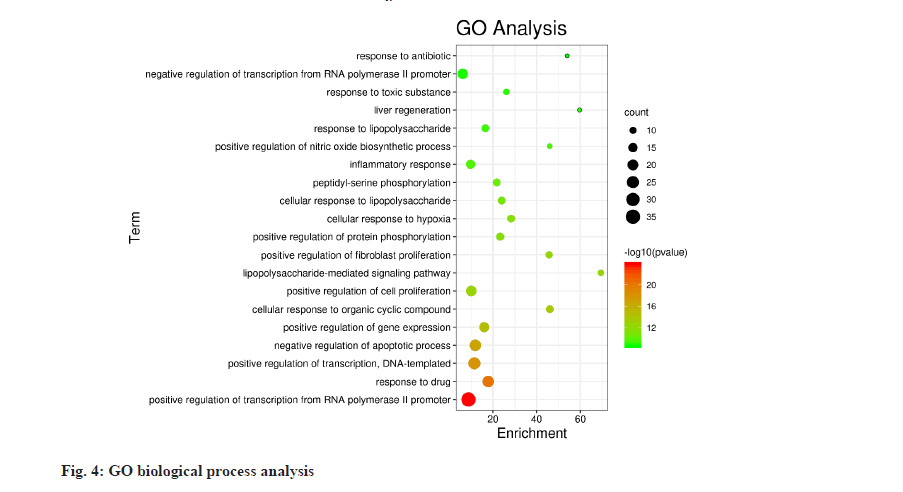
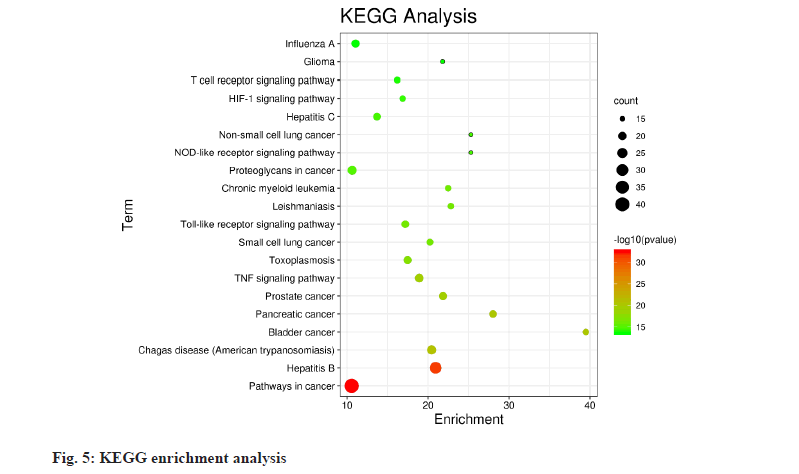
The information of 68 core targets was entered into the DAVID database and the GO biological process analysis and KEGG pathway enrichment analysis were performed on the 68 core targets, and 512 biological processes and 116 pathways were obtained with p≤0.05 as the screening criteria. The top 20 biological processes and pathways were selected according to the order of p-value from smallest to largest and displayed in enrichment bubble plots (fig. 4 and fig. 5), which were drawn by http://www. bioinformatics.com.cn (an online platform for data analysis and visualization). The biological processes involved are mainly positive regulation of Ribonucleic Acid (RNA) polymerase II promoter transcription, positive regulation of transcription, negative regulation of the apoptotic process, inflammatory response, lipopolysaccharidemediated signaling pathway and cellular response to hypoxia. The pathways involved include cancer pathways, hepatitis B, T cell receptor signaling pathway, Nucleotide- Binding Oligomerization Domain (NOD)-like receptor signaling pathway, Hypoxia-Inducible Factor 1 (HIF-1) signaling pathway, Toll-like receptor signaling pathway, etc.
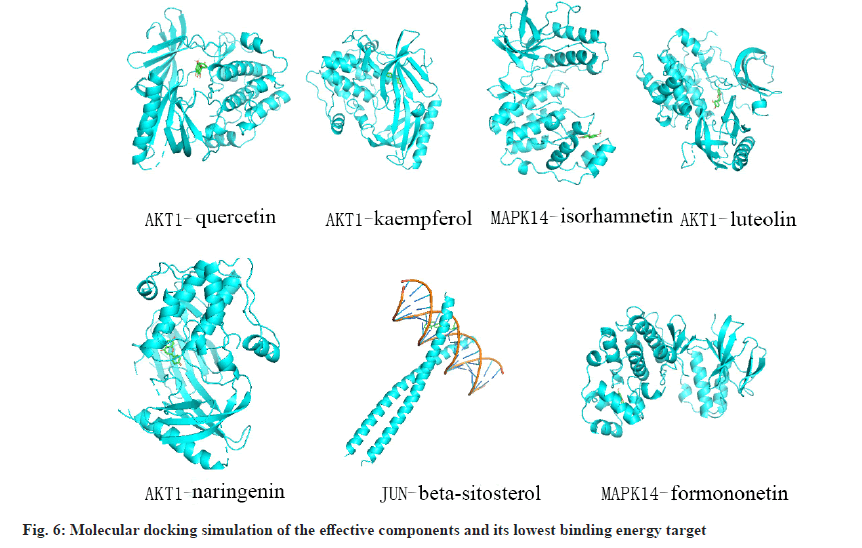
Molecular docking of the 7 main effective components (quercetin, kaempferol, luteolin, naringenin, β-sitosterol, formononetin, isorhamnetin) with the top 10 core targets (AKT Serine/Threonine Kinase 1 (AKT1), Tumor Protein p53 (TP53), Jun Proto-Oncogene, AP-1 Transcription Factor Subunit (JUN), MAPK1, MAPK3, TNF, RELA Proto-Oncogene, NF-κB Subunit (RELA), MAPK8, IL6, MAPK14). It is generally believed that the lower the binding energy, the better the ligand and receptor binding effect. It was found that the lowest binding energy of the binding complexes of the seven effective components and the 10 target proteins were all less than 0 kcal/mol (Table 4) and the molecular docking diagrams of the targets with the best binding effect to the effective components were presented (fig. 6).
| Target | Ingredient | Binding energy (kcal/mol) |
|---|---|---|
| AKT1 | Quercetin | -9.8 |
| AKT1 | Kaempferol | -9.5 |
| AKT1 | Luteolin | -9.8 |
| AKT1 | Naringenin | -9.6 |
| TP53 | Quercetin | -8.8 |
| TP53 | Luteolin | -8.9 |
| JUN | Quercetin | -8.0 |
| JUN | Kaempferol | -8.2 |
| JUN | Luteolin | -8.1 |
| JUN | Beta-sitosterol | -7.0 |
| JUN | Formononetin | -7.4 |
| MAPK1 | Quercetin | -7.5 |
| MAPK1 | Luteolin | -7.8 |
| MAPK1 | Naringenin | -7.4 |
| MAPK3 | Naringenin | -7.7 |
| TNF | Quercetin | -8.8 |
| TNF | Kaempferol | -8.5 |
| TNF | Luteolin | -8.6 |
| RELA | Quercetin | -9.2 |
| RELA | Kaempferol | -9.2 |
| RELA | Luteolin | -9.1 |
| RELA | Naringenin | -8.9 |
| RELA | Isorhamnetin | -8.9 |
| MAPK8 | Kaempferol | -9.2 |
| IL-6 | Quercetin | -7.8 |
| IL-6 | Luteolin | -7.4 |
| MAPK14 | Isorhamnetin | -9.3 |
| MAPK14 | Formononetin | -10.2 |
Table 4: The Binding Energy of the Core targets and their Associated Effective Components
In this study, we analyzed the effective components of six wind medicines, namely, Chuanxiong rhizoma, Ephedrae herba, Bupleuri radix, Asari Radix et Rhizoma, Puerariae lobatae radix, Cinnamomi ramulus and concluded that the key components of the six wind medicines acting on the BBB were quercetin, kaempferol, isorhamnetin, luteolin, formononetin, β-sitosterol and naringenin. These components were found to have a significant protective effect on the BBB. Jin et al.[10] demonstrated that quercetin could improve BBB dysfunction by upregulating the expression of Zonula Occludens-1 (ZO-1), Claudin-5, etc., and decreasing the expression of Matrix Metalloproteinase-9 (MMP-9). Li et al.[11] found that kaempferol could inhibit pro-inflammatory proteins and improve BBB dysfunction in rats with cerebral ischemia-reperfusion. Zhao et al.[12] found that isorhamnetin could improve BBB function and upregulate the expression of occludin, ZO-1 and claudin-5. Zhang et al.[13] found that luteolin could protect the BBB by inhibiting the inflammatory response after BBB injury. Bai et al.[14] found that naringenin could downregulate the expression of Nuclear Factor kappa Light Chain Enhancer of Activated B cells (NF-κB) and MMP-9 and upregulate the expression of claudin-5 to protect the BBB function in rats with cerebral ischemia.
The core targets of wind drugs acting on the BBB screened in this study are all important targets for regulating the BBB and most of them are related to the immune-inflammatory response and apoptosis, such as apoptosis-related proteins (pro- or antiapoptotic) CASP8, CASP3, B-Cell Lymphoma 2 (BCL2), Bcl-2-like protein 1 (BCL2L1), X-linked Inhibitor of Apoptosis Protein (XIAP), etc.; immune inflammation-related proteins TNF, IL-6, IL-1B, IL- 4, IL-10, C-X-C Motif Chemokine Ligand (CXCL) 8, C-C Motif Chemokine Ligand 2 (CCL2), CXCL2, CXCL10, Interferon Gamma (IFNG), CXCL11, etc.; MAPK family, MAPK1, MAPK3, MAPK8, MAPK14; NF-κB family, RELA; MMP9; Vascular Endothelial Growth Factor A (VEGFA), etc. In addition, transcription factor, Signal Transducer and Activator of Transcription 1 (STAT1), HIF1A, sirtuin protein family member, Sirtuin 1 (SIRT1) and other proteins are closely related to the BBB. Current studies have confirmed that inflammation is an important cause of BBB disruption, inflammatory factors can directly damage endothelial cells, IL-6 can make endothelial cell damage by activating NF-κB and TNF-alpha (α) can also induce RELA overexpression and increase BBB permeability[15]. The sirtuin protein family is closely associated with apoptosis and inflammation, and studies have demonstrated that reduced expression of SIRT1 leads to increased permeability of the BBB[16,17]. Production of MMP, especially MMP-9, degrades tight junction and basement membrane proteins and exacerbates BBB disruption, while inhibition of MMP- 9 significantly enhances the protective effect on the BBB[18,19]. MAPK is closely associated with cellular processes such as inflammation and stress responses, and MAPK is an important signaling pathway for BBB disruption and activation of inflammatory responses, and inhibition of the MAPK pathway can significantly improve BBB function[20,21]. Chemokines associated with the immune-inflammatory response are also involved in BBB disruption and CCL2 and CXCL8 are associated with BBB dysfunction[22,23]. Endothelial growth factor induces increased vascular permeability and astrocytes can produce VEGFA to enhance the permeability of the BBB, while inhibition of VEGFA improves BBB function[24]. Studies have confirmed that pro-inflammatory cytokines and chemokines upregulate the expression of VEGFA, thereby exacerbating the disruption of the BBB[25]. When cerebrovascular disease occurs, apoptotic signals including p53, CASP3 and CASP9 are activated, thus promoting apoptosis and disrupting the BBB integrity[26]. GO biological processes include positive regulation of RNA polymerase II promoter transcription, negative regulation of the apoptotic process, lipopolysaccharidemediated signaling pathway, positive regulation of gene expression, inflammatory response, cellular response to hypoxia, positive regulation of nitric oxide biosynthesis process, etc. It mainly involves a series of biological processes such as inflammatory response, apoptosis, signal transduction, lipopolysaccharideinduced response and gene expression. The top 20 signaling pathways involved include cancer pathway, hepatitis B, TNF signaling pathway, toxoplasmosis, Toll-like receptor signaling pathway, NOD-like receptor signaling pathway, HIF-1 signaling pathway, T cell receptor signaling pathway, etc., which are mainly involved in viral or parasitic infection, immuneinflammatory response, apoptosis, etc.
Therefore, the present study confirmed that the wind drug has multi-component, multi-target and multi-pathway characteristics through network pharmacological analysis. It may act on the BBB through anti-inflammatory and anti-apoptotic mechanisms, which is important for the treatment of encephalopathy. However, this study is relatively one-sided and needs to be verified by follow-up experimental studies and so on.
Acknowledgements:
This study is supported by Sichuan Science and Technology Project: 2020YJ0437; the Project of Sichuan Provincial Administration of Traditional Chinese Medicine: 2020JC0139; Luzhou Science and Technology Project: 2020-SYF-30; the Project of National Traditional Chinese Medicine Clinical Research Base: XNYDZYY [2020] No.33; the Southwest Medical University and Affiliated Traditional Chinese Medicine Hospital Joint Program: 2018XYLH-006.
Conflict of interests:
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol 2015;38:2-6.
[Crossref] [Google Scholar] [PubMed]
- Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015;7(1):a020412.
[Crossref] [Google Scholar] [PubMed]
- Ding R, Hase Y, Ameen‐Ali KE, Ndung'u M, Stevenson W, Barsby J, et al. Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and Alzheimer’s disease. Brain Pathol 2020;30(6):1087-101.
[Crossref] [Google Scholar] [PubMed]
- Chen YH, Wu H, Wei JJ. Research progress in assessment methods and mechanisms of neural disease induced by blood-brain barrier injury. Basic Clin Med 2019;39(1):120-4.
- Dong L, Li B, Bai X, Yang S. Correlation between the Xuan-fu of the brain and the blood-brain barrier. J Traditi Chin Med 2013;54(22):1969-71.
- Jiang Y, Pan H, Yan Y. The academic thought and clinical experience of Professor Wang Mingjie in treating encephalopathy from wind theory. Shi-Zhen Guomao 2015;26(03):710-2.
- Wang SQ, Bai X. The effect of opening the Xuan fu method on the bidirectional regulation of the blood-brain barrier after cerebral hemorrhage. Chin J Basic Med Tradit Chin Med 2018;24(11):1530-3.
- Xu P, Tang H, Bai X. Effects of Feng Feng Tong Qiao formula on blood-brain barrier permeability and pericytes with MFSD2A in cerebral ischemic rats. In: Chinese Society for Anatomical Sciences (CSAS). Chinese Society for Anatomical Sciences 2019 Annual Meeting Abstracts Collection. Chinese Society for Anatomical Sciences (CSAS): Chinese Anatomical Society; 2019. p. 2.
- Wang MJ, Huang SF, Luo ZQ, Jiang H, Jiang Y, Wang Z. A new understanding of wind medicine. J Luzhou Med Coll 2011;34(5):570-2.
- Jin Z, Ke J, Guo P, Wang Y, Wu H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am J Transl Res 2019;11(8):4683-95.
[Google Scholar] [PubMed]
- Li WH, Cheng X, Yang YL, Liu M, Zhang SS, Wang YH, et al. Kaempferol attenuates neuroinflammation and blood brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res 2019;1722:146361.
[Crossref] [Google Scholar] [PubMed]
- Zhao JJ, Song JQ, Pan SY, Wang K. Treatment with isorhamnetin protects the brain against ischemic injury in mice. Neurochem Res 2016;41(8):1939-48.
[Crossref] [Google Scholar] [PubMed]
- Zhang JX, Xing JG, Wang LL, Jiang HL, Guo SL, Liu R. Luteolin inhibits fibrillary β-Amyloid (1-40)-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules 2017;22(3):334.
[Crossref] [Google Scholar] [PubMed]
- Bai X, Zhang X, Chen L, Zhang J, Zhang L, Zhao X, et al. Protective effect of naringenin in experimental ischemic stroke: Down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem Res 2014;39(8):1405-15.
[Crossref] [Google Scholar] [PubMed]
- Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-alpha induced NFκB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine 2012;57(2):269-75.
[Crossref] [Google Scholar] [PubMed]
- Ham III PB, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol 2017;157:92-116.
[Crossref] [Google Scholar] [PubMed]
- Stamatovic SM, Martinez-Revollar G, Hu A, Choi J, Keep RF, Andjelkovic AV. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis 2019;126:105-16.
[Crossref] [Google Scholar] [PubMed]
- Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: Friend and foe for ischemic stroke. J Neuroinflammation 2019;16(1):1-24.
[Crossref] [Google Scholar] [PubMed]
- Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 2016;10:56.
[Crossref] [Google Scholar] [PubMed]
- Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin M, et al. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int J Mol Sci 2016;17(9):1418.
[Crossref] [Google Scholar] [PubMed]
- Sun J, Nan G. The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. J Mol Neurosci 2016;59(1):90-8.
[Crossref] [Google Scholar] [PubMed]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood–brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab 2006;26(6):797-810.
[Crossref] [Google Scholar] [PubMed]
- Haarmann A, Schuhmann MK, Silwedel C, Monoranu CM, Stoll G, Buttmann M. Human brain endothelial CXCR2 is inflammation-inducible and mediates CXCL5-and CXCL8-triggered paraendothelial barrier breakdown. Int J Mol Sci 2019;20(3):602.
- Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain 2015;138(6):1548-67.
[Crossref] [Google Scholar] [PubMed]
- Yang R, Liu W, Miao L, Yang X, Fu J, Dou B, et al. Induction of VEGFA and Snail-1 by meningitic Escherichia coli mediates disruption of the blood-brain barrier. Oncotarget 2016;7(39):63839-55.
[Crossref] [Google Scholar] [PubMed]
- Kanamaru H, Suzuki H. Potential therapeutic molecular targets for blood-brain barrier disruption after subarachnoid hemorrhage. Neural Regen Res 2019;14(7):1138-43.
[Crossref] [Google Scholar] [PubMed]