- *Corresponding Author:
- C. B. Tripathi
Department of Pharmacology, Government Medical College, Bhavnagar-364 001
E-mail: cbrtripathi@yahoo.co.in
| Date of Submission | 22 July 2012 |
| Date of Revision | 19 February 2013 |
| Date of Acceptance | 28 February 2013 |
| Indian J Pharm Sci 2013;75(2):191-196 |
Abstract
Neuromuscular weakness is often found in patients receiving zidovudine therapy due to mitochondrial damage. Effect of zidovudine was evaluated in indirectly and directly stimulated isolated rat phrenic nerve hemidiaphragm preparations, by cumulative dose response curve with square wave pulses, 0.5 ms duration of 2 Hz at every 10 s. To understand the observed effect of zidovudine, interaction studies were carried out with rocuronium. Dose response curve of rocuronium was compared in the absence and in the presence of 1.2 and 12 mmol/ml zidovudine. In another set of experiment, intact animals were treated with zidovudine 50 and 100 mg/kg for 15 days and phrenic nerve hemidiaphragm was obtained for in vitro experiment. Effect of zidovudine (30 mmol/ml) on twitch responses inhibited by dantrolene 50 μmol/ml, magnesium chloride 8 mmol/ml, rocuronium 10 μmol/ml, succinylcholine 25 μmol/ml and lignocaine 600 μmol/ml was studied. Zidovudine (3-30 mmol/ml) significantly augmented the twitch responses up to 80 and 40% in indirectly and directly stimulated preparations, respectively (P <0.05). Zidovudine did not show significant interaction with rocuronium in any group as per dose response curve and inhibitory concentration 5%, inhibitory concentration 50% and inhibitory concentration 95% of rocuronium. Zidovudine (30 mmol/ml) augmented the twitch responses inhibited by dantrolene, magnesium chloride and rocuronium. It reduced the time for complete block of succinylcholine (P <0.05). Zidovudine affects the neuromuscular transmission. No conclusive interaction between rocuronium and zidovudine was found.
Keywords
Drug interaction, neuromuscular transmission, rocuronium, zidovudine
Highly active antiretroviral therapy (HAART) is used in the treatment of human immunodeficiency virus (HIV) patients. Zidovudine is the basic antiretroviral drug having a place in almost all regimens of HAART. Muscles are the important target for zidovudine‑induced toxicity [1]. Chronically treated animals with zidovudine have shown alteration in skeletal muscle functions [2]. Long term use of zidovudine is reported to cause clinically significant muscle weakness and myopathy in HIV patients [3,4]. The myopathic symptoms often begin after the first 6 months of therapy and include fatigue, muscle weakness and elevation of muscle enzymes. These symptoms occur in at least 17% of patients and are often reversible when zidovudine therapy is discontinued [5]. Histological, ultrastructural, biochemical and molecular studies in humans and experimental animals have suggested that the myopathy induced by zidovudine is due to mitochondrial toxicity [5,6]. This is due to its inhibitory effect on mitochondrial gamma DNA polymerase [4,7]. Zidovudine may affect neuromuscular function due to impairment of mitochondrial metabolism and attention should be paid to its use due to the likelihood of acquiring neuromuscular weakness in intensive care unit [8].
However, no experimental study is performed to observe the effect of zidovudine on neuromuscular transmission and its interaction with neuromuscular blockers. To evaluate these issues, the present study was carried out using isolated rat phrenic nerve hemidiaphragm preparations.
Materials and Methods
After approval from institutional animal ethics committee, Government Medical College, Bhavnagar, India, all the experiments were performed as per the guidelines issued by the Committee for the Purpose of Control and Supervision on Experiments on Animals, India. Albino Wistar rats (200‑300 g) of either sex were procured from the central animal house of the institute. They were housed in standard transparent polypropylene cages under controlled room temperature (24±2°; relative humidity 60‑70%) in 12‑12 h light‑dark cycle. Animals were fed with food pellets and water ad libitum. Food was withdrawn 12 h before the experiments.
Zidovudine (Cadila Pharmaceuticals Ltd., Ahmedabad, India), dantrolene (Sigma Aldrich, Germany) and magnesium chloride (Qualigen Fine Chemicals, Mumbai, India) were dissolved in double distilled water to get a final concentration of 20, 1 and 10 mg/ml, respectively. Vials of rocuronium (10 mg/ml, Neon Laboratories Ltd., Mumbai, India), lignocaine (20 mg/ml, 2% w/v, 30 ml, Hindustan Laboratories Ltd., Barauni, India) and succinylcholine (50 mg/ml, Healthcare Pvt. Ltd., Mumbai, India) were used with appropriate dilutions in the physiological salt solution (PSS). Chemicals used for the preparation of PSS were of analytical reagent grade.
Rat phrenic nerve hemidiaphragm preparation
Rats were sacrificed and left hemidiaphragm with attached phrenic nerve was dissected as described by Bulbring [9]. The preparations were mounted on rat phrenic nerve diaphragm electrode in inner organ bath containing 40 ml of double dextrose tyrode solution (containing NaCl 136.9 mM, KCl 2.68 mM, CaCl2 1.36 mM, magnesium chloride 0.98 mM, NaH2PO4 0.32 mM and dextrose 11.1 mM) maintained at a temperature of 37±0.5° and bubbled with oxygen. In directly stimulated preparation, phrenic nerve was removed and only diaphragm was mounted. After initial stabilisation, the preparation was stimulated with square wave pulses of 0.5 ms duration, of 2 Hz at every 10 s using the research stimulator RS 48 (Labotech, Ambala, India; amplitude: 2 V in indirect preparation and 20 V in direct preparation). The isometric muscle contractions were quantified with force displacement transducer and recorded on a student physiograph (Inco Biodevice, Ambala, India) through strain gauge coupler. Contact time for each drug was 3 min. For each group, six experiments were performed.
Effect of zidovudine on contraction of the diaphragm
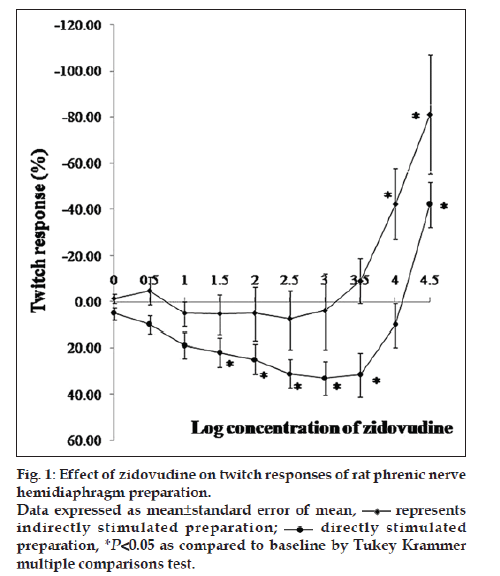
Effect of zidovudine on contractions of the diaphragm induced by indirect and direct stimulation was recorded. Initially, 1 μmol/ml concentration of zidovudine was used; subsequently, incremental doses of 0.5 logarithmic intervals at every 3 min were used till a final concentration of 30 mmol/ml was reached. Thus, cumulative dose response curves (DRCs) of zidovudine were obtained in indirectly (group 1) and in directly stimulated preparations (group 2).
Interaction studies between zidovudine and rocuronium
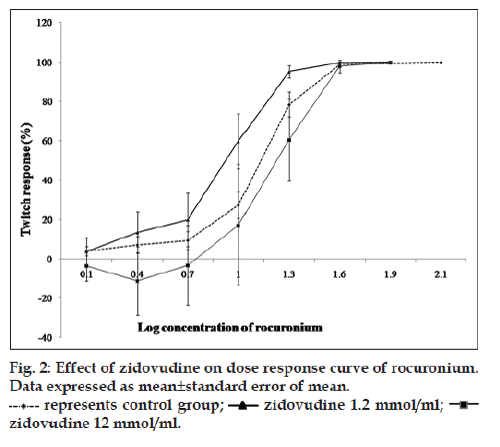
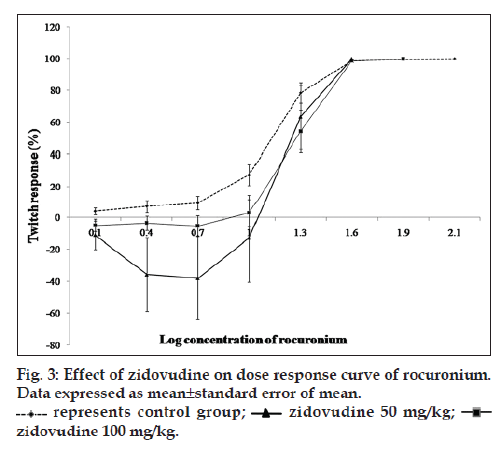
Effect of zidovudine was studied on neuromuscular blockade produced by rocuronium. Rat phrenic nerve hemidiaphragm preparations were mounted to obtain DRC of rocuronium in the absence of zidovudine (group 3) and in the presence of 1.2 (group 4) and 12 mmol/ml of zidovudine (group 5), and in animals treated with 50 mg/kg/day (group 6) and 100 mg/kg/day (group 7) for 15 days. DRC of rocuronium was obtained using its incremental doses at 0.3 logarithmic intervals at every 3 min until the complete blockade is achieved.
Effect of zidovudine on twitch responses inhibited by dantrolene, magnesium chloride, rocuronium, succinylcholine and lignocaine
After obtaining stable twitch responses, rocuronium (10 μmol/ml), succinylcholine (25 μmol/ml), dantrolene (50 μmol/ml), magnesium chloride (8 mmol/ml) and lignocaine (600 μmol/ml) were added to produce neuromuscular blockade in the indirectly stimulated preparation. At around 50% reduction in height of T1, zidovudine (30 mmol/ml) or vehicle (PSS) was added and their effects were compared on twitch responses.
Parameters and statistical analysis
Effect of zidovudine on neuromuscular transmission was studied by comparing the inhibition of twitch response (%) and train of four ratio (T4/T1) at every incremental dose with that of baseline using the repeated measures of analysis of variance (ANOVA) followed by Tukey Krammer multiple comparisons test in cumulative DRC of zidovudine.
Inhibition of twitch responses by rocuronium was calculated and expressed as a percentage from the height of T1 of the train of four responses at every dose as compared to the height of T1 of basal twitch responses. DRC of rocuronium in the absence and in the presence of zidovudine was compared by one‑way analysis of covariance (ANCOVA) using the Statistical Package for the Social Sciences (SPSS) 17 trial version software (IBM corporation, USA). Inhibitory concentration 5% (IC5), 50% (IC50) and 95% (IC95) were calculated from cumulative DRC of rocuronium by employing regression analysis using MasterPlexReaderfit demo version software (MiraiBio Group of Hitachi Solutions America, Ltd). All data were expressed as mean±standard error of the mean. Comparisons of IC5, IC50 and IC95 between the control group and interactions study groups 4 and 5; control group and interactions groups 6 and 7 were performed by ANOVA followed by Dunnett multiple comparisons test. Effect of zidovudine 30 mmol/ml on twitch responses inhibited by dantrolene, magnesium chloride, rocuronium, succinylcholine and lignocaine was calculated in percentage and compared with that of vehicle added group (PSS) by unpaired t‑test.
Percentage of twitch response was calculated using the equation; % twitch response=100–( [A−B/A]×100), where A is the height of T1 in basal twitch response, B is the height of T1, 3 min after the administration of zidovudine or vehicle.
Time required to produce complete block was recorded after administration of zidovudine as compared to vehicle added group, where reversal did not occur. Comparison of time for complete block between vehicle added group and zidovudine added group was performed using unpaired t‑test. P<0.05 was considered significant.
Results
DRC of zidovudine was taken from the concentration of 1 μmol/ml to 30 mmol/ml. Zidovudine in the concentration range of 1 μmol/ml to 1 mmol/ml reduced the twitch responses (without affecting train of four ratio) to the extent of 8 and 30% in indirect and direct stimulation experiments, respectively. In indirectly stimulated preparation, depression of twitch responses was nonsignificant whereas significant in directly stimulated preparation (P<0.05) from the concentration of 3‑30 mmol/ml, zidovudine significantly enhanced the twitch responses (P<0.05). Up to 80% and 40% augmentation of twitch responses were noted with indirect and direct stimulation, respectively (fig. 1). However, this difference of augmentation between indirect and directly stimulated preparations was nonsignificant. Interaction with rocuronium exhibited that at a concentration of zidovudine (1.2 mmol/ml), there was a trend of shift of DRC towards the right whereas at concentration (12 mmol/ml), the trend of the shift was toward the left. However, the shifts were statistically nonsignificant as determined by ANCOVA (fig. 2). Differences between IC5, IC50 and IC95 values of rocuronium in the absence and presence of zidovudine 1.2 and 12 mmol/ml were also nonsignificant (Table 1). In the set of experiment where zidovudine was given in an intact animal for 15 days and phrenic nerve hemidiaphragm was obtained for in vitro experiment, there was the shift of DRC of rocuronium toward right (fig. 3). However, the shift was statistically nonsignificant. As shown in Table 2, IC95 of rocuronium was significantly increased in the dose of 50 mg/kg of zidovudine. Pattern of train of four ratios for rocuronium was not affected in the presence of zidovudine.
| Groups | IC5 | IC50 | IC95 |
|---|---|---|---|
| Control | 6.11 ± 1.27 | 14.24 ± 1.66 | 23.81 ± 2.32 |
| Zidovudine 1.2 mmol | 9.34 ± 2.96 | 14.74 ± 3.33 | 25.10 ± 7.77 |
| Zidovudine 12 mmol | 4.31 ± 0.90 | 11.82 ± 1.12 | 23.15 ± 2.10 |
| P value | 0.2087 | 0.6253 | 0.9589 |
| F (df) (ANOVA) | 1.742 (2.15) | 0.4846 (2.15) | 0.04206 (2.15) |
Table 1: Effect of rocuronium in control and Zidovudine treated groups
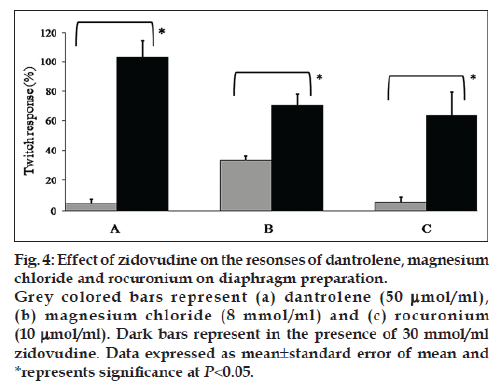
Zidovudine (30 mmol/ml) has significantly augmented the twitch responses inhibited up to 50% by dantrolene (50 μmol/ml), magnesium chloride (8 mmol/ml) and rocuronium (10 μmol/ml) (fig. 4). Block produced by succinylcholine (25 μmol/ml) was not reversed with zidovudine (30 mmol/ml), but it significantly reduced the total time for the complete blockade of succinylcholine as compared to control (238.75±7.7 vs. 192.5±11.3 s; P<0.05). Zidovudine (30 mmol/ml) neither reversed the 50% blockade nor affected the total time for the complete blockade of lignocaine (390±100 vs. 332.5±47.5 s).
Figure 4: Effect of zidovudine on the resonses of dantrolene, magnesium
chloride and rocuronium on diaphragm preparation.
Grey colored bars represent (a) dantrolene (50 μmol/ml),
(b) magnesium chloride (8 mmol/ml) and (c) rocuronium
(10 μmol/ml). Dark bars represent in the presence of 30 mmol/ml
zidovudine. Data expressed as mean±standard error of mean and
*represents significance at P<0.05.
| Groups | IC5 | IC50 | IC95 |
|---|---|---|---|
| Control | 6.11 ± 1.27 | 14.24 ± 1.66 | 23.81 ± 2.32 |
| Zidovudine 50 mg/kg/day for 15 days | 10.01 ± 2.15 | 19.58 ± 2.72 | 34.12 ± 2.49* |
| Zidovudine 100 mg/kg/day for 15 days | 8.64 ± 2.33 | 15.80 ± 2.19 | 24.41 ± 3.53 |
| P value | 0.3888 | 0.252 | 0.0361 |
| F (df) (ANOVA) | 1.007 (2.15) | 1.513 (2.15) | 4.179 (2.15) |
Table 2: Effect of rocuronium in control and Zidovudine treated groups
Discussion
Chronic zidovudine therapy is known to cause clinically significant myopathy and mechanisms proposed for the zidovudine‑induced myopathy are depletion of mitochondrial DNA, oxidative stress, direct inhibition of mitochondrial bioenergetics machinery and mitochondrial depletion of L‑carnitine [4,10]. In the present experiments, zidovudine has augmented the indirect stimulated preparation and exhibited a biphasic response (initial depression followed by augmentation) in the direct stimulated preparation. From the concentration of 1 μmol/ml to 1 mmol/ml, it shown significant depression of twitch responses up to 30% in the direct stimulated preparation suggests its mild inhibitory action on the skeletal muscle. Augmentation of contractions was seen with zidovudine from 3 mmol/ml concentration in both types of the preparations in a dose‑dependent manner. As the augmentation was noted in both indirect as well as directly stimulated preparations, site of action could be on skeletal muscle. However, the higher augmentation of twitch responses on in the indirectly stimulated preparation suggests neuroumuscular component is also involved in its mechanism. It is worth noting that mitochondrial inhibitors are known to augment the miniature end plate potential and neurotransmitter release [11]. Interference with mitochondrial function can lead to release of already sequestered calcium in the mitochondria resulting in a higher calcium concentration in the nerve terminals, which might cause the release of higher amounts of the neurotransmitter [11]. Mefloquine augments the neurotransmitter release by increasing intracellular calcium level due to inhibition of mitochondrial adenosine triphosphate production [12]. Zidovudine is known to affect the mitochondrial function. This may have partly contributed in the augmentation of twitch responses. Observed effect of zidovudine on twitch responses inhibited by dantrolene and magnesium chloride suggests the involvement of calcium related mechanism.
Dantrolene, a direct muscle relaxant produces its action by inhibition of calcium release from sarcoplasmic reticulum. It also markedly depresses the frequency of spontaneous miniature potentials at neuromuscular junction due to its action at intracellular calcium stores in the presynaptic terminals [13]. In the present study, effect of dantrolene was reversed with zidovudine. Caffeine, theophylline and 3,4‑diaminopyridine are known to antagonize the action of dantrolene [13‑15]. Caffeine and theophylline releases calcium from sarcoplasmic reticulum, whereas 3,4‑diaminopyridine inhibits potassium conductance and releases calcium from nerve and muscle membranes [16,17]. Magnesium chloride inhibits entry of extracellular calcium into the nerve terminals and decreases the neurotransmitter release. It also stabilises the postjunctional membrane and reduces excitability of muscle fiber [18]. In the present study, block of magnesium chloride was reversed with zidovudine. From the above observations it can be speculated that zidovudine increases the calcium level at neuromuscular junction and skeletal muscle. Reversal of rocuronium block and potentiated succinylcholine block with zidovudine (30 mmol/ ml) may be explained by its role in facilitation of release of acetyl choline (ACh) by affecting the calcium channel at the neuromuscular junction. However, it requires confirmation through further studies on calcium channels. Local anesthetics produce neuromuscular block by sodium channel blockade. They also promote the shift of nicotinic receptors from a normal state to desensitised state [18]. Zidovudine may not be able to overcome the neuromuscular block produced by lignocaine because of sodium channel blockade or desensitised state of nicotinic receptors.
Trend of shift of DRC was toward the right in both sets of interaction studies except for zidovudine 12 mmol/ml. In the DRC, of zidovudine of indirect stimulation augmentation of twitch responses was observed from 3 to 30 mmol/ml concentration. However, zidovudine 12 mmol/ml did not shift the DRC of rocuronium toward the right. This dose of zidovudine may have cause desensitisation of receptors and potentiate the block of rocuronium by shifting the type of block from competitive to noncompetitive type [19]. Results of pretreated animals with zidovudine were also in line with in vitro addition of zidovudine where 50 mg/kg has shown more effect on DRC of rocuronium than 100 mg/kg. Dose‑dependent effect observed on DRC of rocuronium was not reflected on the interaction studies. Zidovudine 50 mg/kg for 15 days (equivalent human dose 10 mg/kg, a therapeutic dose) nonsignificantly increased IC5 and IC50 and significantly increased IC95 (P<0.05). Zidovudine 100 mg/kg for 15 days (equivalent human dose 20 mg/kg, two times higher therapeutic dose) led to nonsignificant higher values of IC5, IC50 and IC95, but comparatively lower than zidovudine 50 mg/kg group (Table 2). Some differences observed in interaction studies were quite large, statistical significant difference was not found. These observations are interesting, but not conclusive. Confirmatory studies with plasma concentration of zidovudine and level of ACh at the neuromuscular junction in in vivo preparation will be helpful to study the interaction with neuromuscular blocker.
In conclusion, zidovudine affects the neuromuscular transmission. Augmentation of twitch responses is possibly due to its effect on the neuromuscular junction and skeletal muscle. No conclusive interaction between rocuronium and zidovudine is established.
Acknowledgments
We sincerely thank Dr. Unnat Pandit of Cadila Pharmaceuticals Limited, Ahmedabad, India for the gift sample of pure zidovudine. We also thank Dr. V. H. Bhavsar, Professor and Head, Pharmacology, Medical College, Bairagarh, Bhopal, India and Dr. D. C. Tripathi, Professor and Head, Anesthesiology, Government Medical College, Bhavnagar, India for their valuable suggestions.
References
- Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med 1990;322:1098-105.
- Cazzalini O, Lazzè MC, Iamele L, Stivala LA, Bianchi L, Vaghi P, et al. Early effects of AZT on mitochondrial functions in the absence of mitochondrial DNA depletion in rat myotubes. Biochem Pharmacol 2001;62:893-902.
- Lewis W. Mitochondrial dysfunction and nucleoside reverse transcriptase inhibitor therapy: Experimental clarifications and persistent clinical questions. Antiviral Res 2003;58:189-97.
- Scruggs ER, Dirks Naylor AJ. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology 2008;82:83-8.
- Sinnwell TM, Sivakumar K, Soueidan S, Jay C, Frank JA, McLaughlin AC, et al. Metabolic abnormalities in skeletal muscle of patients receiving zidovudine therapy observed by 31P in vivo magnetic resonance spectroscopy. J Clin Invest 1995;96:126-31.
- Arnaudo E, Dalakas M, Shanske S, Moraes CT, DiMauro S, Schon EA. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 1991;337:508-10.
- Neustadt J, Pieczenik SR. Medication-induced mitochondrial damage and disease. Mol Nutr Food Res 2008;52:780-8.
- Maramattom BV, Wijdicks EF. Acute neuromuscular weakness in the intensive care unit. Crit Care Med 2006;34:2835-41.
- Bulbring E. Observation on the isolated phrenic nerve hemidiaphragm preparation of the rat. Br J Pharmacol 1946;1:38-61.
- Dalakas MC, Leon-Monzon ME, Bernardini I, Gahl WA, Jay CA. Zidovudine-induced mitochondrial myopathy is associated with muscle carnitine deficiency and lipid storage. Ann Neurol 1994;35:482-7.
- Alnaes E, Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol 1975;248:285-306.
- McArdle JJ, Sellin LC, Coakley KM, Potian JG, Hognason K. Mefloquine selectively increases asynchronous acetylcholine release from motor nerve terminals. Neuropharmacology 2006;50: 345-53.
- Statham HE, Duncan CJ. Dantrolene and the neuromuscular junction: Evidence for intracellular calcium stores. Eur J Pharmacol 1976;39:141-52.
- Røed A. Caffeine-induced blockade of neuromuscular transmission and its reversal by dantrolene sodium. Eur J Pharmacol 1982;83: 83-90.
- Saito S, Harada M, Yamamoto M, Takagi H, Saito K, Konno Y. Muscle relaxant action of dantrolene sodium in rats. Res Commun Chem Pathol Pharmacol 1993;81:345-54.
- Harvey AL, Marshall IG. The facilitatory actions of aminopyridines and tetraethylammonium on neuromuscular transmission and muscle contractility in avian muscle. Naunyn Schmiedebergs Arch Pharmacol 1977;299:53-60.
- Patel TK, Patel PB, Tripathi CB. Effect of pantoprazole and its interactions with vecuronium on the neuromuscular junction. Indian J Pharmacol 2010;42:36-9.
- Miller RD, editor. Miller’s Anesthesia. 5th ed. New York: Churchill Livingstone; 2000. p. 463-4.
- Jonsson Fagerlund M, Dabrowski M, Eriksson LI. Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology 2009;110:1244-52.