- *Corresponding Author:
- P. Lv
Breast Surgery, Zhoushan Maternal and Child Care Hospital, No.238 Renmin North Road, Zhoushan City, 316300, China
E-mail: lvpingzhous789@126.com
| This article was originally published in a special issue, "Clinical and Experimental Studies on Drug and Intervention Repurposing in China |
| Indian J Pharm Sci 2019:81(4)spl issue1;116-121 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to explore the role of vitamins and paclitaxel on the proliferation of breast cancer cells and to provide a theoretical basis for clinical adjuvant therapy, 50 female patients with triple negative breast cancer were selected as the research subjects. These female patients were diagnosed in Zhoushan Maternal and Child Care Hospital from January 2015 to December 2018. They were then randomly divided into the study group and the control group, with 25 cases in each group. All patients were treated with gemcitabine and cisplatin chemotherapy. On this basis, the study group received adjuvant treatment of paclitaxel combined with vitamin C intravenous drip. According to the patient's medical records and follow-up records, the clinical efficacy, functional status scores, survival time and the level of tumor markers were counted and analyzed. The effect of paclitaxel combined with vitamin C on inhibiting the proliferation of cancer cells in cytological experiments was also investigated. The results showed that there was no significant difference in the treatment efficacy and clinical benefit rate between the two groups. The survival time without disease progression and the total survival time were compared between the two groups. The study group had significantly longer survival time than the control group (p<0.05). After the treatment, the Karnofsky performance status score of the study group was significantly higher than that of the control group (p<0.05). The incidence of side effects of nausea and vomiting, constipation, leukopenia, thrombocytopenia, anemia and abnormal liver function in the study group was significantly lower than that in the control group (p<0.05). The level of tumor markers after treatment was compared between the two groups and the difference was statistically significant (p<0.05). In addition, when paclitaxel was used in combination with vitamin C, the rate of cancer cell inhibition at any concentration was higher than that of paclitaxel and vitamin C alone. Paclitaxel and vitamin C adjuvant therapy for breast cancer could improve the efficiency of clinical treatment, prolong the survival time of patients and reduce the side effects of chemotherapy. In this study, cell experiments further confirmed that paclitaxel combined with vitamin C could inhibit the growth of breast cancer cells, and thus can be used as a theoretical basis for clinical medication.
Keywords
Vitamin C, paclitaxel, breast cancer, cell proliferation, cell apoptosis
Breast cancer is a kind of malignant tumour that poses a serious threat to the health of women worldwide. According to statistics, there were about 1.5 million new breast cancer patients worldwide in 2010 and about 500 000 deaths. The incidence of breast cancer ranks first among all female cancer and is still growing[1]. In recent years, with the development of economy and the improvement of people's living standards, the pace of life is getting faster and faster, and the pressure of work and family is also increasing. The incidence of female breast cancer in China is also on the rise, especially in economically developed coastal cities, such as Shenzhen, Guangzhou, Shanghai and other places, which has seriously threatened women's lives and health[2]. As far as the treatment of breast cancer is concerned, chemotherapy is a commonly used clinical treatment. However, a series of side effects caused by chemotherapy, such as nausea, vomiting, headache, alopecia and systemic symptoms, will bring great harm to patients' physical and mental health[3]. Therefore, there is an urgent need to find a treatment method that not only can ensure the therapeutic effect is not affected, but also reduce the side effects of chemotherapy drugs. Vitamin C or ascorbic acid, is an antioxidant and coenzyme. It cannot be synthesized by the human body and needs to be obtained through food intake. Currently, research has confirmed that adults who consume vitamin C-rich vegetables and fruits every day can effectively prevent the occurrence of various tumours including breast cancer[4].
Paclitaxel (PTX) is one of the most commonly used drugs in the treatment of breast cancer. PTX is extracted from the bark of Taxus brevifolia. Its complex structure is an effective component in the trunk, bark or coniferous part of the tree[5]. The antitumor effect of PTX is to prevent the mitosis of cancer cells by promoting the aggregation of polyproteins and inhibiting their cleavage, thereby affecting cell cycle. PTX is widely used in clinic because of its pronounced cytotoxicity against breast, ovarian, lung, oral and other cancer cells[6]. Therefore, in this study, a retrospective analysis of breast cancer patients using PTX and vitamin C injections was first performed. The effects of combination therapy on breast cancer size, functional status, disease progression (PD), and survival time were analysed. Then, cell experiments were carried out to observe the effect of each PTX and vitamin C, as well as their combination on the growth of breast cancer cells, an attempt to understand the theoretical basis for the low toxicity and high efficiency of breast cancer treatment.
Materials and Methods
Research subjects:
From January 2015 to December 2018, 50 female patients with triple negative breast cancer diagnosed in the Zhoushan Maternal and Child Care Hospital were randomly divided into study group and control group, with 25 cases in each group. Most breast cancer patients in both groups were young under 45 y age and were not menopausal. There was no significant difference in general baseline data between the two groups (p>0.05). There was no significant difference in liver, lung, bone and other metastatic sites between the two groups. All patients received 2 to 8 courses of systemic chemotherapy.
There were four inclusion criteria. The first one was 20 to 65 y old. The second was to meet the diagnostic criteria of triple negative breast cancer. A puncture biopsy or frozen pathology confirmed the pathological type of invasive ductal carcinoma of the breast. Immunohistochemical results showed that oestrogen receptor (ER) <10 % (+), progesterone receptor (PR) <10 % (+), and human epithelial growth factor receptor 2 (Her-2) were (-), which was diagnosed as triple negative breast cancer. The third was that CT, MRI or ultrasonography could confirm the presence of distant or local metastasis, and the target lesion could be measured by imaging examination. The fourth was the incompatibility with other primary malignant tumours.
Intervention methods:
All patients were treated with gemcitabine and cisplatin chemotherapy. The specific methods of chemotherapy were as follows. Twenty eight-day chemotherapy cycle was used. Gemcitabine (1000 mg/m2) as a 30 min intravenous drip was given on d 1 and d 8 of chemotherapy. On the d 2 of chemotherapy, cisplatin (75 mg/m2) was given as a 2 h intravenous infusion. These chemotherapeutic drugs should be applied for 4 to 6 courses according to the patient's own specific conditions. Prior to chemotherapy, all patients were treated with ondansetron and other antiemetic agents to relieve gastrointestinal discomfort, while giving diuretic and hydration treatment. Blood profile was checked regularly during chemotherapy. When white blood cell count fell below 3.5×109/l, especially neutrophils below 1.5×109/l, granulocyte stimulating factor was injected. When platelet count dropped to 80×109/l, interleukin-11 was given. When liver function is abnormal, hepatoprotective drugs such as reduced glutathione and glucurolactone were given.
According to each patient's medical records and followup records, the changes of tumours were statistically analysed, the functional status was scored, and the key information such as the time of recurrence and the time of death were recorded. All patients were evaluated by CT before and after treatment.
Criteria for evaluating efficacy:
According to the evaluation index of breast cancer in the standard for evaluation of solid tumour therapeutic efficacy, the imaging results of patients before and after treatment were compared as a part of tumour evaluation. In this study, after 2 cycles of chemotherapy, imaging examination and detection of tumour-related indicators were graded in 4 levels, complete remission (CR), partial remission (PR), PD and disease stabilization (SD). CR refers to the complete disappearance of all target lesions without new lesions that lasts for at least 4 w. PR refers to the reduction of the total length and diameter of the lesion by more than 30 % compared to the baseline and lasts for at least 4 w. PD is the occurrence of new lesions, or the total length and diameter of lesions increases by more than 20 % compared to the baseline. SD refers to the total length and diameter of lesions that have decreased or increased compared with baseline, but do not meet the standard of PR or PD. CR+PR is effective and CR+PR+SD is a clinical benefit. The effective rate of treatment RR (%) = (CR+PR/total cases)×100, and the clinical benefit rate CBR (%) = (CR+PR+SD/total cases)×100[7,8].
The survival time is the time from the first day of chemotherapy to any cause of death. Functional state score based on the Karnofsky performance status (KPS) score scale was evaluated before and after treatment. The higher the score, the better the patient's health and the more the patient can tolerate the side effects of the treatment. This also indicates that the patient is likely to receive thorough treatment. The lower the score, the worse the patient’s health condition. If the total score is less than 60, several effective anticancer therapies cannot be implemented[9].
It included the detection of serum carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA199), carbohydrate antigen 125 (CA125) and carbohydrate antigen 15-3 (CA153), which were evaluated before and after treatment. Adverse reactions according to the toxicity performance and classification of antineoplastic drugs developed by the National Cancer Research of the United States, the toxicity and side effects of each chemotherapy cycle were evaluated and classified into grade 0-IV.
Cell culture and transfection:
MCF-7 cells were selected as the research object to analyse the effect of PTX combined with vitamin C on apoptosis-related factors of breast cancer cells in vitro. MCF-7 cells of breast cancer were cultured in vitro. The inhibition rates of vitamin C, PTX and PTX+vitamin C on the proliferation of MCF-7 cells were detected using the MTS method, while the IC50 value (concentration necessary to inhibit cell proliferation by 50 %) was calculated. The inhibition rate of tumour cell proliferation was calculated, and the synergistic relationship between the two drugs was calculated using Kim's modified formula.
Statistical analysis:
SPSS20.0 was used to analyse the data. Descriptive statistical method was employed to analyse the general data and problems of patients. The inter-group test was performed using an independent sample t test, and multiple sets of comparisons were analysed by analysis of variance. The measurement data were expressed in the form of mean±standard deviation. The counting data were expressed as a percentage of cases and analysed by x2 test. Multivariate logistic regression analysis was used to analyse the influencing factors, with p<0.05 as the statistical significance.
Results and Discussion
Among the 50 cases of breast cancer investigated in this study, 2 cases of CR, 9 cases of PR, 11 cases of SD and 3 cases of PD were found in the study group. The effective rate of treatment was 44 % and the clinical benefit rate was 88 %. In the control group, there was no CR, 8 cases of PR, 12 cases of SD and 5 cases of PD. The effective rate of treatment was 32 % and the clinical benefit rate was 80 %. There was no significant difference in treatment efficiency and clinical benefit rate between the 2 groups (p>0.05). The short-term clinical effects of both the groups were compared as shown in Table 1.
| Group | Cases | CR | PR | SD | PD | RR | CBR |
|---|---|---|---|---|---|---|---|
| Study group | 25 | 2 | 9 | 11 | 3 | 44 % | 88 % |
| Control group | 25 | 0 | 8 | 12 | 5 | 32 % | 80 % |
| χ2 | - | - | - | - | - | 1.434 | 0.976 |
| P | - | - | - | - | - | 0.203 | 0.239 |
Table 1: Comparison of short-term clinical efficacy between the two groups
In this study, all patients were followed up throughout the study. The median follow-up time was 18 mo. The results showed that 12 patients survived and 13 of whom died in the study group. In the control group, 5 patients survived and 15 died. The median duration of disease-free progression was 6.8 mo and the median overall survival time was 25.3 mo in the study group. The median survival time without PD in the control group was 4.2 mo, while the median overall survival time was 18.5 mo. The survival time without PD and the total survival time were compared between the two groups. The study group was longer than the control group, and the difference was statistically significant (p<0.05). According to statistics, the 3-y survival rate of the study group was 40.0%, which was significantly higher than that of the control group (16 %, p<0.05). The survival rates of the two groups were compared as listed in Table 2. It can be seen that on the basis of routine chemotherapy regimen, supplementary intravenous infusion of PTX combined with vitamin C could improve the internal environment disorder and has a positive effect on improving the chemotherapy. Therefore, after the use of drugs, the clinical efficacy of patients has been significantly improved, and the survival time has been significantly prolonged.
| Group | ≤1 year | ≤2 years | ≤3 years | ≤4 years |
|---|---|---|---|---|
| Study group | 5 | 7 | 10 | 3 |
| Control group | 2 | 19 | 4 | 0 |
Table 2: Comparison of survival situation between the two groups of patients
Before treatment, there was no significant difference in KPS scores between the 2 groups of patients, which is comparable. After 2 courses of treatment, the KPS scores of the patients in the study group were significantly higher than those before treatment, and the difference was statistically significant (p<0.05). There was no significant difference in the KPS score between the control group (p>0.05). The KPS scores of the patients in the study group were significantly higher than those in the control group, and the difference was statistically significant (p<0.05). The functional status scores of the 2 groups of patients before and after treatment are tabulated in Table 3. This shows that through the adjuvant treatment of PTX and vitamin C, the overall functional status of the patient was significantly improved compared to before treatment. The KPS score increased from the previous 76.30±4.22 to 88.39±4.31. The patient was already at a non-dependent level and could take care of himself. Usually, if the KPS score is greater than 80 points, it indicates that the overall state of the patient is better and the survival period is longer.
| KPS | Before treatment | After treatment | P |
|---|---|---|---|
| Research group | 76.30±4.22 | 88.39±4.31 | 0.000 |
| Control group | 76.45±4.09 | 78.59±4.79 | 0.271 |
| P | >0.05 | 0.000 | - |
Table 3: Comparison of functional status scores before and after treatment in two groups of patients
Both groups of patients received a combination chemotherapy of cisplatin and gemcitabine. The adverse reactions of the patients were mainly digestive tract discomfort and myelosuppression. According to statistics, most of the adverse reactions in the study group were mild, mostly in grade I to II, including digestive tract reactions such as nausea and vomiting (5 cases), constipation (3 cases), leukopenia (10 cases), thrombocytopenia (9 cases), anaemia (9 cases), abnormal liver function (5 cases), alopecia (3 cases), rash (2 cases) and peripheral neurotoxicity (1 case). The main adverse reactions of grade III to IV are leukopenia, anaemia and thrombocytopenia. The adverse reactions of patients in the control group were higher than those in the study group, with a slightly higher severe degree, but most of them were in grade I-II. The incidence of side effects such as nausea and vomiting, constipation, leukopenia, thrombocytopenia, anaemia and abnormal liver function in the study group was significantly lower than in the control group (p<0.05). There were no significant differences in the incidence of other adverse reactions such as alopecia, rash and peripheral neurotoxicity between the two groups (p>0.05). This also demonstrates that the intravenous drip of vitamin C combined with PTX can effectively reduce the side effects of chemotherapy on the basis of routine chemotherapy regimen. The Table 4 displays the occurrence of adverse reactions to chemotherapy in both groups.
| Symptom | Study group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | |
| Anemia | 2 | 3 | 2 | 1 | 4 | 7 | 3 | 1 |
| Leukopenia | 3 | 4 | 2 | 1 | 5 | 8 | 4 | 1 |
| Thrombocytopenia | 2 | 3 | 3 | 1 | 4 | 4 | 4 | 1 |
| Vomit | 2 | 2 | 1 | 0 | 5 | 5 | 2 | 0 |
| Constipation | 2 | 1 | 0 | 0 | 4 | 2 | 0 | 0 |
| Alopecia | 2 | 1 | 0 | 0 | 4 | 2 | 1 | 0 |
| Liver dysfunction | 3 | 2 | 0 | 0 | 7 | 3 | 0 | 0 |
| Peripheral neurotoxicity | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Rash | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
Table 4: Comparison of adverse reactions between the two groups of patients
Before treatment, there was no significant difference in the level of tumour markers between the two groups (p>0.05). After 2 courses of treatment, the levels of tumour markers including CEA, CA199, CA153 and CA125 in the study group were significantly lower than those before treatment (p<0.05). There was no significant difference in tumour marker levels between the control group and those before treatment (p>0.05). The level of tumour markers after treatment was compared between the two groups, and the difference was statistically significant (p<0.05). It can be seen that conventional chemotherapy has a lower lethality for cancer cells that have developed drug resistance, while adjuvant PTX and vitamin C treatment can effectively improve the effect of chemotherapy and significantly reduce tumour-related indicators, which is more conducive to anticancer treatment. Table 5 depicts the comparison of tumour markers before and after treatment in both groups.
| Tumor markers | Research group | P | Control group | P | ||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |||
| CEA | 120.3±49.2 | 25.3±9.6 | 0.000 | 119.3±48.0 | 121.3±51.3 | 0.40 |
| CA199 | 34.8±20.3 | 17.3±7.2 | 0.000 | 36.5±21.5 | 38.5±16.9 | 0.42 |
| CA153 | 170.6±88.2 | 53.4±24.5 | 0.000 | 172.6±79.8 | 166.4±70.5 | 0.82 |
| CA125 | 37.3±17.8 | 19.4±12.4 | 0.000 | 36.1±18.6 | 62.5±15.4 | 0.34 |
Table 5: Comparison of tumor markers before and after treatment in two groups of patients
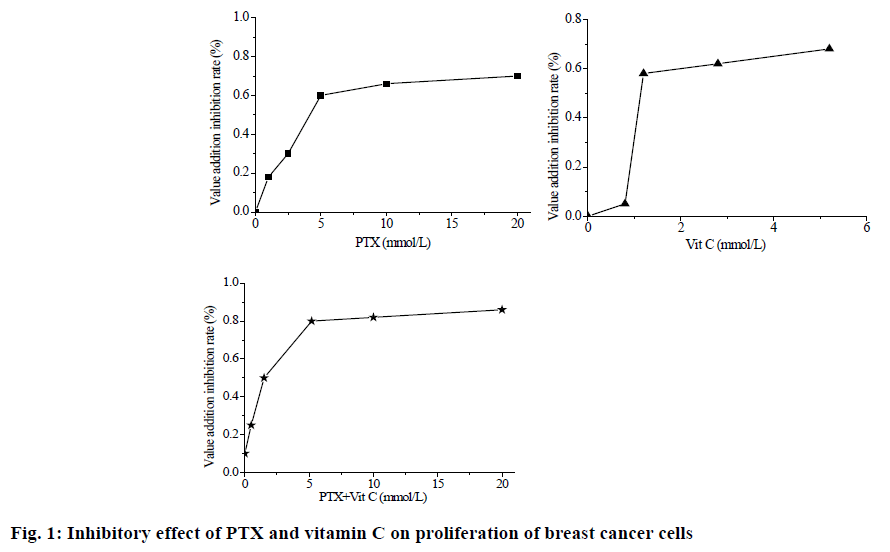
In this study, the following points were discovered through cell experiments. The inhibitory effect of PTX alone on the proliferation of breast cancer cells was dose-dependent, IC50 was 8.5 mM. When vitamin C is used alone, the concentration of vitamin C exceeded 1.25 mM, the inhibitory effect of vitamin C on the proliferation of breast cancer cells is dose-dependent. When IC50 is less than 1.25 mM, the inhibition rate of cancer cells is significantly reduced, regardless of the concentration of IC50=2.5 mM. When used in combination with PTX and vitamin C, the inhibitory effect of PTX and vitamin C on the proliferation of breast cancer cells was dose-dependent, IC50=2.7 mM. Moreover, the inhibition rate of cancer cells is higher than that of each PTX and vitamin C at the same concentration. The picture illustrates the inhibitory effect of PTX and vitamin C on the proliferation of breast cancer cells (fig. 1).
From the cause analysis, cell proliferation and apoptosis are complex multi-gene regulatory processes, which are also affected by both proto-oncogenes and antioncogenes. Mitochondrial microenvironment changes play a key role in the process of apoptosis. Bcl-2 family proteins can regulate the release of IMS as well as the opening and closing of PT channels, thereby regulating cell apoptosis. The combination of vitamin C and PTX can induce apoptotic signals more easily, thus enhancing the killing effect of PTX on breast cancer cells.
At present, the clinically common treatments for breast cancer include surgery, chemotherapy, radiotherapy, endocrine therapy and molecular targeted therapy. The clinical efficacy of breast cancer has been significantly improved by a combination of methods. With increasing attention to breast cancer as a new treatment method, neoadjuvant therapy has gradually been applied to the clinical as a common anticancer treatment program. The neoadjuvant therapy is to reduce the staging of tumours and gradually reduce the size of original large tumour. It not only reduces the patient's trauma area, but also helps patients regain the chance of operation when the tumour progresses to an advanced stage and cannot be operated. In addition, as a key treatment for breast cancer, the side effects of various chemotherapeutic drugs also follow. Most patients will have different degrees of nausea and vomiting, constipation, alopecia and other symptoms, which will cause great harm to patients. The neoadjuvant therapy can alleviate these adverse reactions to some extent.
PTX is one of the most representative chemotherapeutic drugs for breast cancer. As a first-line and second-line drug for adjuvant chemotherapy for breast cancer, PTX has achieved satisfactory results. Especially for some advanced malignant breast cancer, the clinical effect is very significant in the treatment of metastatic cases. As an anticancer drug, PTX targets microtubules and acts on cell cycle. It can play a role in G2 and M phases of cell division, inhibiting the formation of spindles as well as the function of microtubules during cell mitosis, and ultimately inhibiting the apoptosis of cancer cells. As an oxidant, vitamin C can inhibit the activity of hyaluronidase and the hydrolysis of collagen, hence, enhancing the stability of collagen structure and inhibiting the metastasis of cancer cells. In addition, vitamin C can mediate oxidative stimulation around cancer cells to produce hydrogen peroxide, thereby killing cancer cells.
Based on the above theoretical basis and previous research results as well as the basis of conventional chemotherapy, the combination of PTX and vitamin C intravenous drip therapy for advanced breast cancer patients was used in this study to analyse its clinical efficacy and long-term prognosis. The results showed that PTX and vitamin C static adjuvant therapy for breast cancer can enhance the effect of chemotherapy, improve the efficiency of clinical treatment, prolong the survival of patients and reduce the side effects of chemotherapy drugs. In this study, further cell experiments confirmed that PTX combined with vitamin C can inhibit the growth of breast cancer cells, and thus can be used as a theoretical basis for clinical medication.
References
- Chowdhury P, Nagesh PKB, Khan S, Hafeez BB, Chauhan SC, Jaggi M, et al. Development of polyvinylpyrrolidone/paclitaxel self-assemblies for breast cancer. Acta Pharm Sin B 2018;8(4):602-14.
- Vernieri C, Milano M, Mennitto A, Maggi C, Ferrari B, Rinaldi L, et al. Antitumor activity and safety profile of weekly carboplatin plus paclitaxel in metastatic breast cancer: a ten-year, monocentric, retrospective study. Breast Cancer Res Treat 2017;165(2):1-9.
- El-Azem N, Pulido-Moran M, Ramirez-Tortosa CL, Quiles JL, Cara FE, Sanchez-Rovira P, et al. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur J Nutr 2018;(1):1-9.
- Sootichote R, Thuwajit P, Singsuksawat E, Warnnissorn M, Yenchitsomanus PT, Ithimakin S, et al. Compound A attenuates toll-like receptor 4-mediated paclitaxel resistance in breast cancer and melanoma through suppression of IL-8. BMC Cancer 2018;18(1):231.
- Asaduzzaman M, Constantinou S, Min H, Gallon J, Lin ML, Singh P, et al. Tumour suppressor EP300, a modulator of paclitaxel resistance and stemness, is downregulated in metaplastic breast cancer. Breast Cancer Res Treat 2017;163(3):461-74.
- Lewis CW, Jin Z, Macdonald D, Wei W, Qian XJ, Choi WS, et al. Prolonged mitotic arrest induced by Wee1 inhibition sensitizes breast cancer cells to paclitaxel. Oncotarget 2017;8(43):73705-22.
- Liu T, Sun H, Liu S, Yang Z, Li L, Yao N, et al. The suppression of DUSP5 expression correlates with paclitaxel resistance and poor prognosis in basal-like breast cancer. Int J Med Sci 2018;15(7):738-47.
- Rajappa S, Joshi A, Doval DC, Batra U, Rajendranath R, Deo A, et al. Novel formulations of docetaxel, paclitaxel and doxorubicin in the management of metastatic breast cancer. Oncol Lett 2018;16(3):3757-69.
- Kim SB, Dent R, Im SA, Espié M, Blau S, Tan AR, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017;18(10):1360.