- *Corresponding Author:
- Xiangyan Chen
Department of Pediatrics, Baiyun Hospital Affiliated to Guizhou Medical University, Guiyang, Guizhou Province 550014, China
E-mail: 15211516866@163.com
| Date of Received | 18 December 2021 |
| Date of Revision | 05 October 2022 |
| Date of Acceptance | 08 August 2023 |
| Indian J Pharm Sci 2023;85(4):1150-1156 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect and molecular mechanism of total flavonoids of Astragalus on the apoptosis and inflammation of alveolar epithelial cells induced by tumor necrosis factor alpha. Human alveolar epithelial cells were divided into control group, tumor necrosis factor alpha group, tumor necrosis factor alpha+trifluoroacetic acid-low, tumor necrosis factor alpha+trifluoroacetic acid-medium, tumor necrosis factor alpha+trifluoroacetic acid-high group, tumor necrosis factor alpha+anti-microRNA-NC group, tumor necrosis factor alpha+microRNA-221 inhibitor group, tumor necrosis factor alpha+trifluoroacetic acid-high+microRNA-NC group, tumor necrosis factor alpha+trifluoroacetic acid-H+microRNA-221 mimic group. Real-time fluorescence quantitative polymerase chain reaction was used to detect the expression of microRNa-221; (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was used to detect the cell proliferation inhibition rate; flow cytometry to detect the apoptosis of alveolar epithelial cells; enzyme-linked immunosorbent assay to detect the levels of interleukin-1 beta, I interleukin-6 and interleukin-10. In tumor necrosis factor alpha-induced alveolar epithelial cells, the expression of microRNA-221 was increased, the cell proliferation inhibition rate and apoptosis rate were increased, the levels of interleukin-1 beta, interleukin-6 were increased and the level of interleukin-10 was decreased (p<0.05). After treatment with medium and high concentrations of total flavones of Astragalus, the expression of microRNA-221 in alveolar epithelial cells induced by tumor necrosis factor alpha was decreased, cell proliferation inhibition rate and apoptosis rate were decreased, interleukin-1 beta and interleukin-6 levels were decreased and interleukin-10 was increased (p<0.05). Inhibition of microRNA-221 expression can inhibit tumor necrosis factor alpha induced apoptosis and inflammation of alveolar epithelial cells. Overexpression of microRNA-221 can reverse the effect of total flavonoids of Astragalus on apoptosis and inflammation of alveolar epithelial cells induced by tumor necrosis factor alpha. Total flavones of Astragalus inhibited tumor necrosis factor alpha induced apoptosis and inflammation of alveolar epithelial cells by down-regulating microRNA-221.
Keywords
Total flavones, Astragalus, microRNA-221, alveolar epithelial cells, apoptosis, inflammation
Acute lung injury is one of the clinical respiratory diseases, and its main pathophysiological characteristics are the infiltration and apoptosis of inflammatory cells, inhibiting inflammatory factor production, and reducing the apoptosis of alveolar epithelial cells are effective measures for its treatment[1]. Studies have found that Traditional Chinese Medicine (TCM) can be used to treat acute lung injury, and based on the pathogenesis of acute lung injury, TCM and monomers of TCM with significant efficacy can be selected, which can guide clinical medication[2,3]. Total flavones of Astragalus, a major active ingredient of antioxidation extracted from TMC Astragalus, have been studied and found to have immunomodulatory and anti-inflammatory effects[4]. Studies have reported that different concentrations of total flavones of Astragalus have protective effects against high glucose induced endothelial cell injury in human umbilical vein line[5]. Total flavones of Astragalus may play a protective role in rats with cerebral ischemia-reperfusion injury by inhibiting the level of oxidative stress, reducing inflammatory response, anti-apoptosis and other pathways[6]. In addition, total flavones of Astragalus can prevent the development of radiation pneumonitis in mice through antioxidant mechanisms[7]. However, the effect and mechanisms of total flavones of Astragalus on Tumor Necrosis Factor-Alpha (TNF-α) induced alveolar epithelial cell apoptosis and inflammation remain unclear. Studies have reported increased microRNA (miR-221) expression levels in asthmatic patients; silencing miR-221 attenuates airway inflammation in asthma models[8]. Inhibition of miR-221 can inhibit lipopolysaccharide induced secretion of inflammatory factors and inhibit cell apoptosis in alveolar epithelial cells by targeting Adiponectin Receptor 1 (AdipoR1)[9]. Inhibition of miR-221 attenuates lipopolysaccharide induced inflammation in mice with acute lung injury by enabling Suppressor of Cytokine Signaling 1 (SOCS1)/Nuclear Factor-Kappa B (NF-κB) signaling pathway inactivation[10]. Above studies demonstrated that miR-221 was involved in regulating the inflammatory response and lung injury in alveolar epithelial cells. Therefore, this experiment aimed to investigate whether total flavones of Astragalus affect TNF-α induced alveolar epithelial cell apoptosis and inflammation by regulating miR-221.
Materials and Methods
Materials:
Human alveolar epithelial cells were purchased from ScienCell, United States of America (USA); Dulbecco's Modified Eagle Medium (DMEM) medium was purchased from Hyclone™, USA; recombinant human TNF-α was purchased from Invivogen, USA; total flavones of Astragalus were purchased from Shanghai Ziqibio Co., Ltd.; miRNA reverse transcription kit was purchased from GeneCopoeia, USA; miRNA fluorescence qualitative Polymerase Chine Reaction (qPCR) kit was purchased from Shanghai Genepharma Co., Ltd.; (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) kit, Annexin V-Fluorescein Isothiocyante (FITC)/Propidium Iodide (PI) apoptosis detection kit were purchased from Wuhan Amyjet Scientific Inc.; protein extraction kits were purchased from Wuhan Chundu Biotechnology Co., Ltd.; Interleukin-1 (IL)-1 beta (β), IL-6 and IL-10 assay kits were purchased from Beijing Baio Leibo Technology Co., Ltd.
Treatment and grouping of cells:
Human alveolar epithelial cells were cultured in DMEM supplemented with 10 μg/l of TNF-α treated human alveolar epithelial cells as TNF-α group, normal cultured cells as the control group; cells were treated with 5 μg/ml, 10 μg/ml, 50 μg/ml Trifluoroacetic Acid (TFA) and 10 μg/ml of TNF-α treated human alveolar epithelial cells, denoted TNF-α+TFA-Low (TFA-L), TNF-α+TFA-Medium (TFA-M) and TNF-α+TFA-High (TFA-H) groups; 6 h after transfection of anti-miR-NC and miR- 221 inhibitor into human alveolar epithelial cells, cells were treated in 10 μg/l of TNF- α, denoted as TNF-α+anti-miR-NC group and TNF-α+miR-221 inhibitor group; 6 h after transfection of miR-NC and miR-221 mimic into human alveolar epithelial cells, cells were treated in 10 μg/ml TFA and 10 μg/l TNF-α, denoted as TNF-α+TFA-H+miR-NC, and TNF-α+TFA-H+miR-221 mimic groups.
Real time-qPCR (RT-qPCR) to detect miR-221 expression levels:
Total Ribonucleic Acid (RNA) from alveolar epithelial cells of each group was extracted and reverse transcribed into complementary Deoxyribonucleic Acid (cDNA) using miRNA reverse transcription kit, PCR amplified using U6 as internal reference, and relative expression was calculated using the 2-ΔΔCt method.
Cell proliferation inhibition rate by MTT assay:
The cells in each group were cultured for 48 h, operated according to the instructions of the kit, dimethyl sulfoxide was added after the reaction of MTT solution for 4 h, and the absorbance (Optical Density (OD)) value at 490 nm was measured by a microplate reader
Cell proliferation inhibition rate=OD value of experimental group/OD value of control group.
Apoptosis by flow cytometry:
Cells cultured for 48 h were collected, manipulated according to the kit instructions, and finally the apoptosis rate was detected by upper flow cytometry.
Detection of protein expression by Western blot:
Total cellular proteins were extracted and separated by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), followed by membrane shift, blocking, addition of primary antibodies for B-cell lymphoma protein 2 (Bcl-2) and (Bcl-2)-associated X (Bax) Bax overnight at 4°, incubation with secondary antibodies for 1 h at room temperature, development, imaging and analysis of the gray levels of protein bands using image J, and calculation of protein expression levels using Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as an internal reference.
Detection of IL-1β, IL-6 and IL-10 levels by Enzyme- Linked Immunosorbent Assay (ELISA):
The supernatants were harvested after 48 h of cell culture in each group, which was performed according to the kit operation.
Statistical analysis:
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) 20.0 software, and the measurement data that conformed to normal distribution were expressed as mean±standard deviation (x̄ ±s), t-test was used for comparison between two groups, oneway Analysis of Variance (ANOVA) was used for comparison between multiple groups, and Least Significant Difference (LSD)-test was used for comparison between two groups. p<0.05 was taken as statistically significant.
Results and Discussion
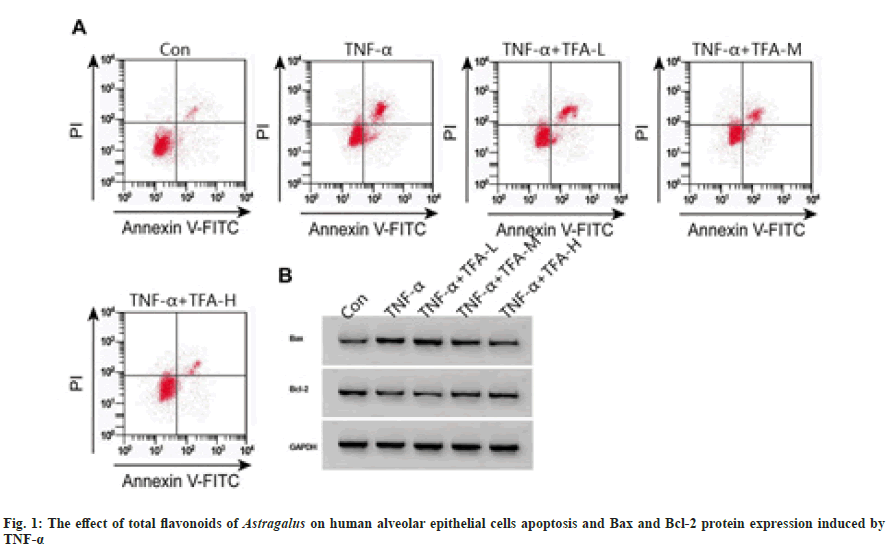
Compared with the control group, the expression levels of miR-221 in TNF-α group increased, the cell proliferation inhibition rate and apoptosis rate increased, the expression level of Bax increased, and the expression level of Bcl-2 decreased in all groups (p<0.05); compared with TNF-α group, miR-221 expression levels in TNF- α+TFA-M, TFA-H group gradually decreased, cell proliferation inhibition rate and apoptosis rate gradually decreased, Bax expression level gradually decreased and Bcl-2 expression level gradually increased (p<0.05) as shown in fig. 1 and Table 1.
| Group | miR-221 | Inhibition rate (%) | Apoptosis rate (%) | Bax | Bcl-2 |
|---|---|---|---|---|---|
| Control | 1.00±0.00 | 0.00±0.00 | 6.44±0.36 | 0.11±0.01 | 0.68±0.06 |
| TNF-α | 5.65±0.16a | 64.53±3.75a | 25.18±1.02a | 0.79±0.06a | 0.15±0.01a |
| TNF-α+TFA-L | 5.62±0.19 | 65.13±3.68 | 25.02±1.71 | 0.80±0.07 | 0.16±0.02 |
| TNF-α+TFA-M | 4.32±0.14bc | 47.49±3.47bc | 21.03±1.46bc | 0.56±0.04bc | 0.35±0.03bc |
| TNF-α+TFA-H | 2.30±0.11bcd | 31.27±3.10bcd | 14.19±1.17bcd | 0.27±0.03bcd | 0.53±0.04bcd |
| F | 386.277 | 224.697 | 127.132 | 129.23 | 121.886 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with the control group, ap<0.05; compared with the TNF-α group, bp<0.05; compared with TNF-α+TFA-L group, cp<0.05 and compared with TNF-α+TFA-M group, dp<0.05
Table 1: Effect of Total Flavones of Astragalus on Cell Viability and Apoptosis (x̄±s, n=3)
Compared with the control group, IL-1β and IL-6 levels increased, and level of IL-10 decreased in TNF-α group (p<0.05); compared with TNF-α group, IL-1β and IL-6 levels were gradually decreased in TNF-α+TFA-M, TFA-H group, and the levels of IL-10 were gradually increased (p<0.05) as shown in Table 2.
| Group | IL-1β (pg/ml) | IL-10 (pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|
| Control | 143.08±9.76 | 971.79±50.73 | 380.30±22.80 |
| TNF-α | 633.92±35.51a | 143.44±14.11a | 1339.56±57.59a |
| TNF-α+TFA-L | 631.80±34.16 | 152.05±19.71 | 1319.28±67.94 |
| TNF-α+TFA-M | 504.29±22.69bc | 366.38±24.06bc | 979.77±42.96bc |
| TNF-α+TFA-H | 316.36±20.17bcd | 720.82±44.08bcd | 537.16±36.55bcd |
| F | 197.908 | 351.817 | 250.29 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with the control group, ap<0.05; compared with the TNF-α group, bp<0.05; compared with TNF-α+TFA-L group, cp<0.05 and compared with TNF-α+TFA-M group, dp<0.05
Table 2: The Effect of Total Flavonoids of Astragalus on Inflammatory Factors
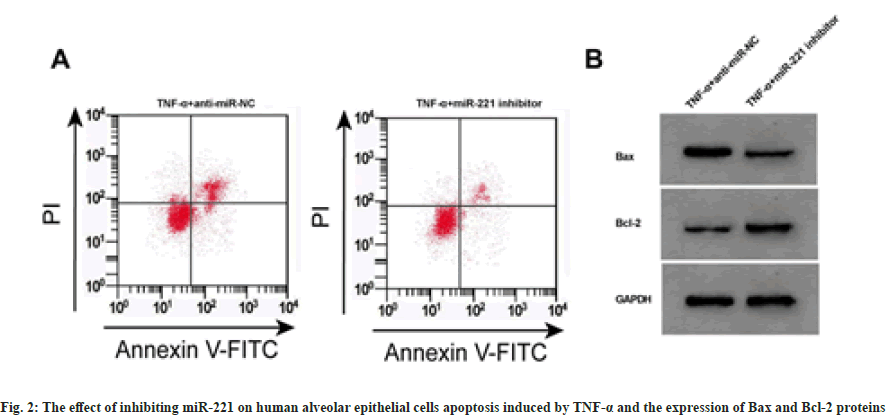
Compared with TNF-α+anti-miR-NC group, the expression levels of miR-221 were decreased in TNF-α+miR-221 inhibitor group, and the cell proliferation inhibition rate and apoptosis rate were decreased, while the expression levels of Bax were decreased, Bcl-2 were increased, and the levels of IL-1β and IL-6 were decreased, and the levels of IL-10 were increased (p<0.05) as shown in fig. 2 and Table 3.
| Group | miR-221 | Inhibition rate (%) | Apoptosis rate (%) | Bax | Bcl-2 | IL-1β (pg/ml) | IL-10 (pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|---|---|---|---|---|
| TNF-α+anti-miR-NC | 5.67±0.16 | 64.69±4.03 | 25.00±1.53 | 0.81±0.07 | 0.13±0.01 | 640.12±39.07 | 150.19±19.28 | 1337.12±50.69 |
| TNF-α+miR-221 inhibitor | 1.88±0.09a | 24.50±2.45a | 10.02±0.75a | 0.17±0.02a | 0.58±0.05a | 232.76±18.18a | 854.47±48.57a | 453.10±31.25a |
| t | 35.759 | 14.686 | 15.227 | 15.227 | 15.286 | 16.373 | 23.343 | 25.713 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with TNF-α+anti-miR-NC, ap<0.05
Table 3: The Effect of Inhibiting miR-221 on Cell Activity, Apoptosis and Inflammatory Factors (x̄±s, n=3)
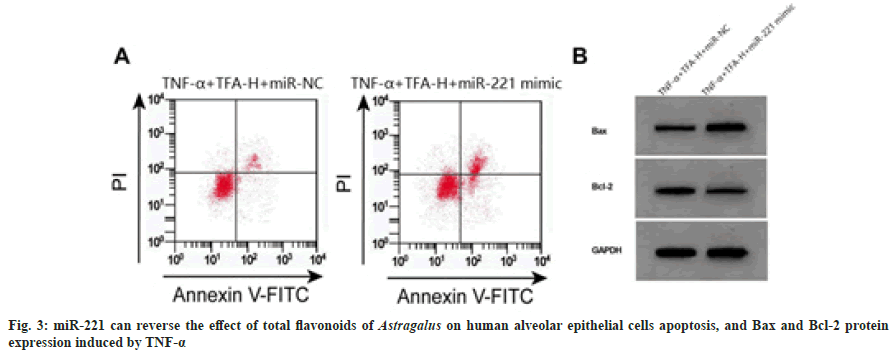
Compared with TNF-α+TFA-H+miR-NC group, the miR-221 expression levels increased in TNF- α+TFA-H+miR-221 mimic group, cell proliferation inhibition and apoptosis rates increased, Bax expression levels increased, and Bcl-2 expression levels decreased (p<0.05) as shown in fig. 3 and Table 4.
| Group | miR-221 | Inhibition rate (%) | Apoptosis rate (%) | Bax | Bcl-2 |
|---|---|---|---|---|---|
| TNF-α+TFA-H+miR-NC | 2.30±0.13 | 31.35±3.10 | 14.34±1.02 | 0.27±0.02 | 0.53±0.05 |
| TNF-α+TFA-H+miR-221 mimic | 4.85±0.14a | 55.08±3.52a | 23.73±1.25a | 0.72±0.06a | 0.23±0.02a |
| t | 23.118 | 8.763 | 10.081 | 12.324 | 9.679 |
| p | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 |
Note: Compared with TNF-α+TFA-H+miR-NC group, ap<0.05
Table 4: miR-221 can Reverse the Effect of Total Flavonoids of Astragalus on Cell Viability and Apoptosis (x̄±s, n=3)
Compared with TNF-α+TFA-H+miR-NC group, the levels of IL-1β and IL-6 increased in TNF- α+TFA-H+miR-221 mimic group, and the levels of IL-10 decreased (p<0.05) as shown in Table 5.
| Group | IL-1β (pg/ml) | IL-10 (pg/ml) | IL-6 (pg/ml) |
|---|---|---|---|
| TNF-α+TFA-H+miR-NC | 316.73±22.89 | 725.27±49.86 | 542.44±31.78 |
| TNF-α+TFA-H+miR-221 mimic | 568.62±30.04a | 259.49±27.83a | 1191.01±55.34a |
| t | 11.552 | 14.129 | 17.603 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with TNF-α+TFA-H+miR-NC group, ap<0.05
Table 5: miR-221 can Reverse the Effect of Astragalus Flavonoids on Inflammatory Factors (x̄±s, n=3)
Acute lung injury is a critical illness with high lethality that is common in the clinic, and TCM has achieved significant efficacy in preventing and treating acute lung injury disease processes, and studying its pharmacological effects on acute lung injury protection may provide a reference for effective clinical treatment and drug development[11,12]. Studies have reported that total flavones of Astragalus may inhibit inflammatory factor secretion from osteoarthritic chondrocytes by down-regulating NF-κB signaling pathway[13]. Total flavones of Astragalus can reduce serum induced endothelial cell apoptosis in uremic patients[14]. Total flavones of Astragalus have shown protective effects in mice with viral myocarditis[15]. Total flavones of Astragalus alleviate experimental autoimmune encephalomyelitis through inhibition of activation of the c-Jun N-terminal kinase (JNK)/ Protein kinase B (AKT)/NF-κB signaling pathway and inflammatory responses[16]. Above studies indicate that total flavones of Astragalus have anti-inflammatory effects. TNF-α induced alveolar epithelial cells for this experiment were used to establish the injury model, and the results showed that the TNF-α induced proliferation inhibition rate of alveolar epithelial cells increased, the apoptosis rate increased, and the levels of IL-1β and IL-6 increased and the levels of IL-10 decreased; IL-1β and IL-6 are pro-inflammatory factors, IL-10 is an anti-inflammatory factor, with high expression of IL-1β and IL-6 and low expression of IL-10 in a rat acute lung injury model[17]. Therefore, the present experimental results indicated that the model was established successfully. In this experiment, different concentrations of total flavones of Astragalus were used to treat TNF-α induced alveolar epithelial cells, the results showed that treatment with total flavones of Astragalus at medium and high concentrations reduced the cell proliferation inhibition rate and apoptosis rate, decreased the expression level of Bax, increased the expression level of Bcl-2, and decreased levels of IL-1β and IL-6 and increased levels of IL-10; studies have shown that high expression of IL-10 can inhibit the inflammatory response to pneumonia in rats with acute lung injury[18]. These results indicate that certain concentrations of total flavones of Astragalus can inhibit TNF-α induced alveolar epithelial cell apoptosis and inflammatory response.
Studies have reported that miR-221 can promote apoptosis of human retinal microvascular endothelial cells under high glucose conditions[19]. miR-221 is involved in airway epithelial cell damage in asthma by targeting Sirtuin 1 (SIRT1) [20]. The results of this experiment showed that the miR-221 expression levels were increased in TNF-α induced alveolar epithelial cells; however, after inhibition of miR-221 expression, apoptosis rate of alveolar epithelial cells induced by TNF-α was decreased, the levels of IL-1β and IL-6 decreased, and the IL-10 level was increased; it showed that inhibition of miR-221 expression suppressed TNF-α induced alveolar epithelial cell apoptosis and inflammatory response. Furthermore, in this experiment, we found that treatment with total flavones of Astragalus decreased miR-221 expression, whereas miR-221 over-expression reversed the effects of total flavones of Astragalus on TNF-α induced alveolar epithelial cell apoptosis and inflammatory response.
In conclusion, total flavones of Astragalus inhibits TNF-α induced alveolar epithelial cell apoptosis and inflammatory response by down-regulating miR-221.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhu K, Zhao H. Recent advances in study of mechanism of acute lung injury. J Med Theory Pract 2018;31(19): 2872-925.
- Li X, Yang A. Pathogenesis of acute lung injury and considerations on treatment based on syndrome differentiation in traditional Chinese medicine. Chin J Integr Tradit Western Med Intensive Crit Care 2018;25(1): 9-14.
- Li K, Song L, Liu J. Recent advances in TCM for the prevention and treatment of acute lung injury. Asia Pac Tradit Med 2019;15(6):175-7.
- Li J, Xu L, Sang R, Yu Y, Ge B, Zhang X. Immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus by regulating NF-κB and MAPK signalling pathways in RAW 264.7 macrophages. Pharmazie 2018;73(10):589-93.
[Crossref] [Google Scholar] [PubMed]
- Tang J, Xu J, Yuan X. Protective effects of different concentrations of total flavones of Astragalus on high glucose induced endothelial cell injury in HUVECs. Res Integr Tradit Chin Western Med 2020;12(3):160-4.
- Ma L, Li H, Sun L. Effect of total flavones of Astragalus on oxidative stress, inflammation and apoptosis in rats with cerebral ischemia-reperfusion injury. Chin Tradit Patent Med 2019;41(8):1811-5.
- Wang J. Prevention of acute radiation pneumonitis in mice by total flavones of Astragalus. Unive Jinan 2011;6(9):13-4.
- Qin H. Effect of miRNA-221 on airway inflammation in bronchial asthma and regulation of mast cell secretion. Nanjing Med Univ 2013;9:2-6.
- Qi S, Feng Xi. Effects of miR-221 targeting AdipoR1 gene on LPS-induced A549 inflammatory secretion and apoptosis in alveolar epithelial cells. Chin J Immunol 2020;36(5):538-54.
- Wang T, Jiang L, Wei X, Dong Z, Liu B, Zhao J, et al. Inhibition of miR-221 alleviates LPS-induced acute lung injury via inactivation of SOCS1/NF-κB signaling pathway. Cell Cycle 2019;18(16):1893-907.
[Crossref] [Google Scholar] [PubMed]
- Li X, Chen Y, Bai X. Study advances in mechanism of traditional Chinese medicine in acute lung injury/acute respiratory distress syndrome. J Liaoning Univ Tradit Chin Med 2017;19(1):166-70.
- Ling Y, Zhang P, Wang A, Jin H. Pharmacological effects and research progress of natural products on protection against acute lung injury. Food Drug 2018;20(1):53-60.
- Ren W, Jiao Y, Zhang J. Effect of total flavones of Astragalus on oxidative stress and inflammatory factor secretion in osteoarthritic chondrocytes. J Zhengzhou Univ Med Sci 2019;54(4):603-6.
- Su J, Li J, Liu T, Ji Y, Li K, Wu R, et al. The effects of total flavonoids of Astragalus on the apoptosis of vascular endothelial cells induced by serum of uremia patient. Int J Lab Med 2014;35(18):2427-9.
- Niu X, Zhao Y, Tao X. An experimental study on the protective effects of total flavones of Astragalus on viral myocarditis in mice by inhibiting endoplasmic reticulum stress. J Clin Cardiol 2015;31(2):129-32.
- Yang L, Han X, Xing F, Wu H, Shi H, Huang F, et al. Total flavonoids of Astragalus attenuates experimental autoimmune encephalomyelitis by suppressing the activation and inflammatory responses of microglia via JNK/AKT/NFκB signaling pathway. Phytomedicine 2021;80:153385.
[Crossref] [Google Scholar] [PubMed]
- Han H, Hong D, Chen Y. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury in rats. Basic Clin Med 2019;39(5):696-700.
- Chen H, Xiong B. Effects of IL-10 on lung UGRP1 and inflammatory factors in LPS induced acute lung injury rats. Med J Natl China 2020;30(3):194-7.
- Chen B, Wu L, Cao T, Zheng HM, He T. miR-221/SIRT1/Nrf2 signal axis regulates high glucose induced apoptosis in human retinal microvascular endothelial cells. BMC Ophthalmol 2020;20(1):300.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Song Y, Rong W, Fan L, Cai Y, Qu Q, et al. miR-221 participates in the airway epithelial cells injury in asthma via targeting SIRT1. Exp Lung Res 2018;44(6):272-9.
[Crossref] [Google Scholar] [PubMed]