- Corresponding Author:
- B. Antony
R and D Laboratory, Arjuna Natural Extracts Ltd., P. B. No. 126, Bank Road, Alwaye-683 101, 1Little Flower Hospital and Research Centre, Angamaly-683 572, India.
E-mail: benny@arjunanatural.com
| Date of Submission | 18 February 2005 |
| Date of Revision | 24 September 2005 |
| Date of Acceptance | 12 July 2006 |
| Indian J Pharm Sci, 2006, 68 (4): 437-441 |
Abstract
Emblica officinalis , commonly known as Indian gooseberry ( Amla ), is found to be effective for the reversal of dyslipidemia and intima-media thickening and plaque formation in the aorta in hypercholesterolaemic rabbits. In this study, cholesterol powder (100 mg/kg body weight) was administered orally to healthy NZ white rabbits for 4 mo to induce hypercholesterolaemia; and thereafter, amla extract was given in two doses (10 mg and 20 mg/kg/d orally) for 4 mo. Fasting lipid profile was done monthly and also at the end of treatment. After sacrificing the animals, tissue cholesterol (liver, heart and kidney) and 3-hydroxy-3-methylglutaryl-Coenzyme A reductase activity of liver were estimated and part of aorta and myocardium were processed for histological studies. Feeding of amla extract (10 mg and 20 mg/kg) for 4 mo reversed these changes and the lumen of the aorta became normal as in the normal control group. Reversal of dyslipidemia and atheromatous plaques achieved by amla extract seems to be brought about by a number of factors, such as its ability to prevent low-density lipoprotein oxidation, its antioxidant action, besides decreasing synthesis of cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Coenzyme A reductase activity and elevating high-density lipoprotein level to enhance reverse cholesterol transport.
Emblica officinalis Gaertn; Euphorbiaceae is an herbal plant widely used in many of the indigenous medicinal preparations against a variety of diseases [1]. It is also known as Phyllanthus emblica Linn, Indian gooseberry or Amlaki and is used in Ayurveda as a cardiotonic. Earlier studies on the effect of Emblica officinalis in hypercholesterolaemia and atherosclerosis were limited to its effect on total serum cholesterol and low-density lipoprotein (LDL) cholesterol [2-5]. A recent study conducted in rats showed that flavonoids from E. officinalis effectively reduced lipid levels in serum and tissues and had significant inhibitory effect on hepatic 3- hydroxy-3-methylglutaryl-Coenzyme A (HMG CoA) reductase activity [6]. The tannoid principles of fruits of E. officinalis have been reported to exhibit antioxidant activity in vitro and in vivo. In a study conducted in rats, it was seen that emblicanin-A- and emblicanin-B- enriched fraction of fresh juice of Emblica fruits show antioxidant activity in ischemia-reperfusion-induced oxidative stress in rat hearts [7]. The E. officinalis fruit increases cardiac glycogen levels andaqueous extract of decreases serum glutamic oxaloacetic transaminase, glutamic pyruvic transaminase and LDL in rats having induced myocardial necrosis [8].
The present study confirms and further extends the earlier observations on the beneficial effects of the Emblica extract in hypercholesterolaemia and atherosclerosis. Two significant findings, not reported before, of the present study are (1) enhancement of the beneficial high-density lipoprotein (HDL) cholesterol and (2) reduction in the intima-media thickening in atherosclerotic animals.
Materials and Methods
Fresh fruits of Emblica officinalis were collected from Coimbatore during August 2001 and pharmacognostically identified by comparing with voucher specimen number AE - HBRS - 011. The chemicals used for the reactions were purchased from Merck. Pure cholesterol was collected from Sigma Chemical Co, USA.
Preparation of extract
Fresh fruits of amla (E. officinalis) (100 kg) were cleaned, crushed, deseeded and refluxed with 50% methanol for 2h. The resultant pulp was cooled and filtered. The filtrate was collected and the residue was refluxed again with 50% methanol. The process was repeated twice and all the filtrates were pooled. The combined washings were subjected to membrane filtration (reverse osmosis) to obtain a concentrate of ca.40% TDS (total dissolved solids). The retentate was dried using agitated thin film dryer under controlled conditions to obtain a dried powdered extract (yield 4.2%) containing polyphenols (30%) of light greenish to dark greenish brown, free flowing, slightly hygroscopic powder.
The extract solution for oral feeding was prepared by adding 15 g sorbitol, 10 g propylene glycol, 25 g amla extract and 50 ml water (25%). This was kept as stock solution and diluted with distilled water as per required concentration. Control solution was prepared in the same manner without adding amla extract.
Induction of hypercholesterolaemia and treatment with Amla extract
The study was conducted at Little Flower Hospital and Research Centre, Angamaly, Kerala. Ethical clearance for the handling of experimental animals was obtained from the Institutional Ethical Committee constituted for the purpose. Male NZ white rabbits weighing 1.3-1.6 kg were used for the experiment. They were housed in a temperature-controlled room (at 28+1°) in clean stainless steel cages with ‘12 h light and 12 h dark’ cycles and fed with normal diet and water ad libitum.
After the acclimatization period of 6-8 days, rabbits were divided into four groups, each group comprising six rabbits. One group (group A) was kept as normal control and the remaining 3 groups (groups B, C and D) were made hypercholesterolaemic by feeding cholesterol powder 100 mg/ kg body weight daily along with diet for 4 mo4. At the end of 4 mo, group B was kept as untreated hypercholesterolaemic control and given vehicle. The groups C and D were orally administrated amla extract in the dosage of 10 and 20 mg/kg/day respectively for 4 mo. Body weight and food intake were recorded every 15 d.
Biochemical and histological studies
Before starting the experiment, the lipid profile of all the animals was done (serum cholesterol, HDL, VLDL, LDL and triglycerides). This was repeated every month. Serum total cholesterol and HDL were estimated by enzymatic method [9,10]. Triglyceride was estimated by GPO-PAP method [11]. LDL and VLDL were calculated using Friedwald formula [12].
‘At the end of 8 mo, the animals were sacrificed under 30 mg/kg pentobarbitone anaesthesia (i.v.). Tissues (liver, aorta, heart and kidney) were collected and examined for gross macroscopic changes. Histology of the tissues was studied after fixing the tissues in 10% formalin [13]. Cholesterol content of liver, kidney and heart were estimated [14,15]. HMG CoA reductase activity of liver was also estimated [16].
Statistical analysis
One-way ANOVA with repeated measures was used to analyse the variance over a period of time. Intergroup comparisons were made using one-way ANOVA. The level of significance was set at P < 0.05. Dunnet multiple comparison test was used to compare the baseline values with periodical observations. Post-ANOVA comparison in intergroup analysis was performed using Turkey-Kramer multiple comparison test. Paired ‘t’ test was used to compare the biochemical parameters both before and after the experiment.
Results and Discussion
Oral feeding of cholesterol resulted in a fivefold increase in total serum cholesterol of rabbits (Table 1). Four months of amla treatment almost reversed the effect and the serum cholesterol level at the end of the treatment period was near normal. A similar effect was noted in the case of LDL cholesterol and TG levels. The extent of lipid changes between the two groups fed different doses of the extract was not significantly different, indicating that a dosage of 10 mg/kg body weight of extract was sufficient to reverse the hypercholesterolaemic condition in rabbits.
| Serum parameter | Baseline | After induction | Treatment period | |||
|---|---|---|---|---|---|---|
| 1 mo | 2 mo | 3 mo | 4 mo | |||
| Total cholesterol | 55.8±5.0 | 56.7±2.7 | 53.4±3.3 | 53.3±3.3 | 54.2±0 | 52.8±1.8 |
| Group A | ||||||
| Group B | 50.0±2.7 | *229.2±3.2 | 220.3±2.8 | **200.8±3.2 | **185.31±1.9 | **164.3±3.6 |
| Group C | 48.3±4.3 | *228.3±1.9 | **163.6±5.8 | **88.9±5.1 | **74.6±3.4 | **63.3±2.9 |
| Group D | 52.5±3.2 | *230.8±3.2 | **178.0±4.4 | **95.0±1.9 | **72.9±2 | **58.3±2.9 |
| HDL cholesterol | 8.3±0 | 8.57±0 | 8.2±0 | 8.3±0 | 8.3±0 | 8.4±0.1 |
| Group A | ||||||
| Group B | 8.7±0.8 | 9.3±1.0 | 7.8±0.8 | 8.8±0.8 | 8.9±1.1 | 8.3±0 |
| Group C | 8.8±1.4 | 8.1±0.6 | 8.2±0 | 8.9±1.0 | 10.1±1.8 | *11.2±1.1 |
| Group D | 8.8±0.8 | 8.5±1.2 | 8.6±0.8 | 8.8±0.8 | 10.1±1.4 | *10.6±1.1 |
| LDL-cholesterol | 39.2±4.9 | 39.7±2.6 | 38.6±5.8 | 36.5±4.1 | 37.6±0.5 | 36.6±1.6 |
| Group A | ||||||
| Group B | 32.4±1.8 | *195.7±4.3 | 187.4±1.9 | **169.8±3.7 | **154.1±3.2 | **136.2±4.4 |
| Group C | 32.4±4.7 | *193.7±2.2 | **137.5±5.2 | **67.5±5.6 | **54.3±2.7 | **43.0±2.3 |
| Group D | 33.6±3.4 | *195.9±2.4 | **153.8±5.2 | **73.8±2.9 | **53.4±1.4 | **39.3±2.1 |
| Triglycerides | 41.3±3.9 | 42.2±3.9 | 40.4±2.2 | 41.3±2.8 | 39.7±2.7 | 39.7±2.7 |
| Group A | ||||||
| Group B | 44.6±2.1 | *134.8±4.4 | 126.0±5.8 | **111.53±5.4 | **121.9±41.4 | **98.7±4.6 |
| Group C | 43.8±0.61 | *133.6±2.1 | **88.5±2.2 | *62.67±2.31 | **51.3±2.2 | **45.3±2.3 |
| Group D | 44.6±2.1 | *132.6±3.3 | **85.6±4.8 | **61.0±2.0 | **47.1±1.9 | **42.7±2.3 |
Group A was normal and groups B, C and D were given cholesterol powder in the dose of 100 mg/kg for 4 mo; after this, groups C and D were given in the dose of 10 and 20 mg/kg respectively for 4 mo. Values are represented as mean±SD. *Significant increase (P < 0.05) **Significant decrease (P < 0.05)
Table 1: Effect Of Oral Administration Of Amla Extract On Serum Lipid Profile In Hypercholesterolaemic Rabbits
Feeding amla extract produced a significant and steady increase in the beneficial HDL concentrations in NZ rabbits after an initial lag period. This result is highly significant in that low HDL-C is now considered as the most significant risk factor for atherosclerosis [17,18].
After inducing hypercholesterolaemia, 4 mo of treatment with amla extract caused an increase in HDL level both in groups C and D compared to the initial level. Treatment with amla extract for 4 mo showed a significant (P < 0.01) increase in HDL level, viz, to 35 and 28% in groups C and D respectively, compared to the corresponding untreated controls (Table 2).
| Category | Group | 1 month | 2 month | 3 month | 4 month |
|---|---|---|---|---|---|
| Total | B×C | 26↓ | *56↓ | *60↓ | *62↓ |
| Cholesterol | B×D | 20↓ | *53↓ | *62↓ | *65↓ |
| HDL | B×C | 5↑ | 2↑ | **14↑ | **35↑ |
| B×D | 10↑ | 0 | **14↑ | **28↑ | |
| LDL | B×C | 27↓ | *60↓ | *65↓ | *68↓ |
| B×D | 19↓ | *57↓ | *65↓ | *71↓ | |
| Triglyceride | B×C | *30↓ | *44↓ | *57↓ | *54↓ |
| B×D | *32↓ | *45↓ | *61↓ | *57↓ |
Percentage of increase or decrease is expressed by comparing with untreated hypercholesterolaemic control. *Significant decrease, **Significant increase, Figures in numeric are in percentage.
Table 2: Effect Of Amla Extract On Lipid Profile In Rabbits (Percentage Of Increase Or Decrease)
One-month treatment with amla extract caused a significant (P < 0.05) decrease in LDL level in groups C and D, while there was no significant decrease in group B (untreated hypercholesterolaemic control). Continuous oral feeding of amla extract further decreased LDL cholesterol level and 4 mo of treatment decreased LDL cholesterol to a highly significant (P < 0.001) level in both groups C and D compared to untreated hypercholesterolaemic control (group B) (Tables 1 and 2).
The triglycerides and VLDL also decreased to a significant level (P < 0.01) in the amla extract treated group. One-month treatment with amla extract produced a significant (P < 0.05) decrease in triglyceride level. After 4 mo of treatment with amla extract, the levels of VLDL and triglycerides were within normal range (Tables 1 and 2).
Cholesterol content of liver was significantly (P < 0.05) higher in group B compared to normal control group. Cholesterol of heart and kidney was also higher in group B compared to normal but not significantly (P > 0.05). In treatment groups (C and D), liver cholesterol was found to decrease significantly (P < 0.05) and was almost normal, as in group A. The cholesterol content of heart and kidney was also lower in treatment groups compared to group B but not significantly. The HMG CoA reductase activity was reduced in the treatment groups (C and D) compared to untreated control group (B) (Table 3).
| Cholesterol | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| content (mg/g) | ||||
| Liver | 9.4±0.2 | *14.6±0.5 | **9.5±0.1 | **9.6±0.2 |
| 35%↓ | 35%↓ | |||
| Heart | 7.3±0.1 | 8.5±0.2 | 7.5±0.1 | 7.4±0.1 |
| 4%↓ | 4%↓ | |||
| Kidney | 5.6±0.1 | 6.6±0.2 | 5.6±0.1 | 5.7±0.0 |
| 1%↓ | 1%↓ | |||
| Liver HMG CoA/ | 1.0±0.2 | 0.9±0.2 | **1.5±0.6 | ** 1.3±0.1 |
| Mevalonatea | 40%↓ | 31%↓ |
Group A was normal and groups B, C and D were given cholesterol powder in the dose of 100 mg/kg for 4 mo; after this, groups C and D were given Reducerol TM in the dose of 10 and 20 mg/kg for 4 mo. Values are represented as mean±SD. *Significant increase (P < 0.05), **Significant decrease (P < 0.05). The percentage of increase or decrease showed in groups C and D was calculated by comparing to group B. aActivity is expressed as ratio of HMG CoA/Mevalonate, i.e., the higher the ratio, lower the activity.
Table 3: Effect of Oral Administration of Amla Extract on Tissue Cholesterol and Hmgcoa Reductase Activity In Hypercholesterolaemic Rabbits
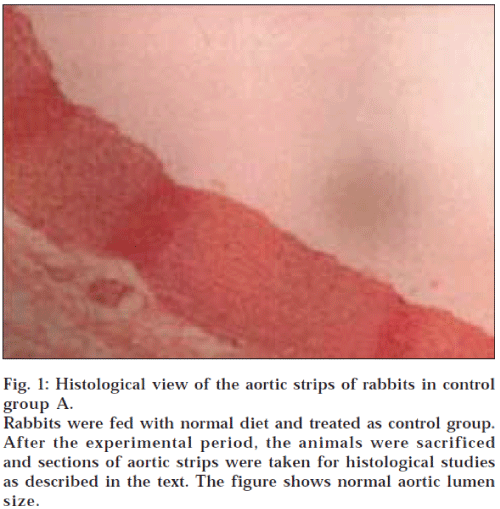
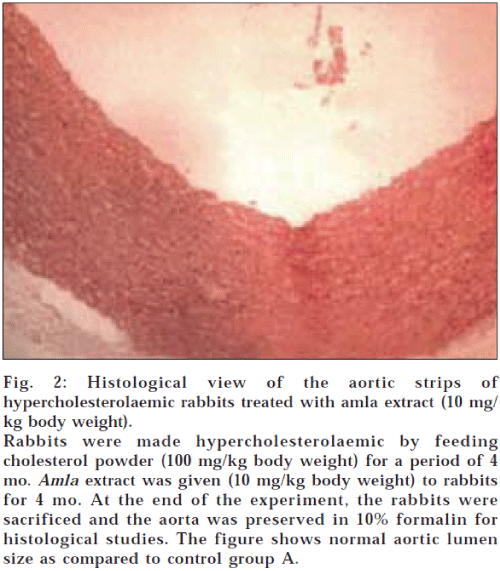
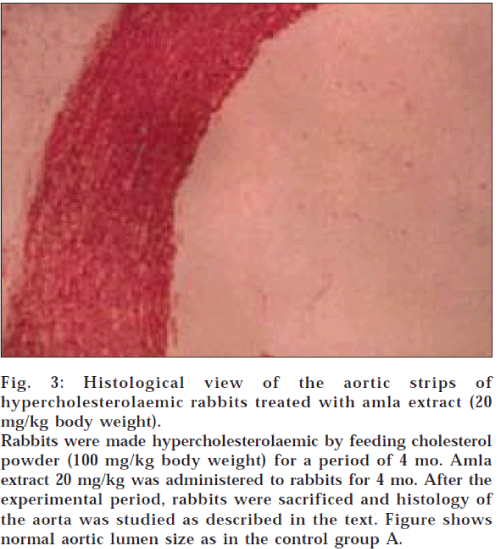
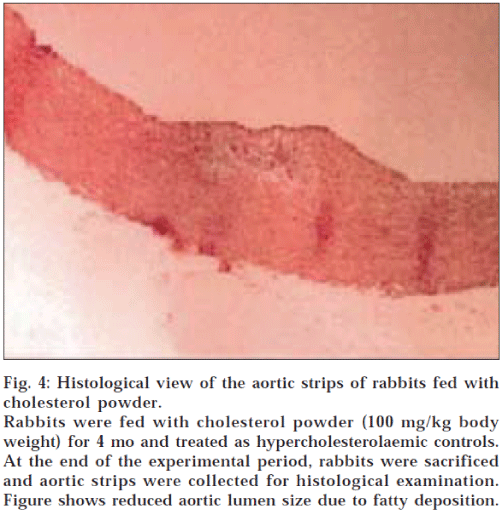
Histological examination of aortic strips of control group rabbits was normal with normal intima, media and adventitia (fig. 1). In the treatment groups (C and D) (10 mg/kg and 20 mg/kg), aortic lumen diameter was of normal size (figs. 2 and 3), as in the control group (fig. 1) and atheromatous lesion was also absent; whereas lumen size of aorta in group B (fig. 4) was reduced due to fatty deposition and atheromatous plaque formation.
Fig. 1: Histological view of the aortic strips of rabbits in control group A.
Rabbits were fed with normal diet and treated as control group. After the experimental period, the animals were sacrificed and sections of aortic strips were taken for histological studies as described in the text. The figure shows normal aortic lumen size.
Fig. 2: Histological view of the aortic strips of hypercholesterolaemic rabbits treated with amla extract (10 mg/ kg body weight).
Rabbits were made hypercholesterolaemic by feeding cholesterol powder (100 mg/kg body weight) for a period of 4 mo. Amla extract was given (10 mg/kg body weight) to rabbits for 4 mo. At the end of the experiment, the rabbits were sacrificed and the aorta was preserved in 10% formalin for histological studies. The figure shows normal aortic lumen size as compared to control group A.
Fig. 3: Histological view of the aortic strips of hypercholesterolaemic rabbits treated with amla extract (20 mg/kg body weight).
Rabbits were made hypercholesterolaemic by feeding cholesterol powder (100 mg/kg body weight) for a period of 4 mo. Amla extract 20 mg/kg was administered to rabbits for 4 mo. After the experimental period, rabbits were sacrificed and histology of the aorta was studied as described in the text. Figure shows normal aortic lumen size as in the control group A.
Fig. 4: Histological view of the aortic strips of rabbits fed with cholesterol powder.
Rabbits were fed with cholesterol powder (100 mg/kg body weight) for 4 mo and treated as hypercholesterolaemic controls. At the end of the experimental period, rabbits were sacrificed and aortic strips were collected for histological examination. Figure shows reduced aortic lumen size due to fatty deposition.
Oral feeding of cholesterol powder to experimental rabbits produced significant elevation in the levels of serum total cholesterol, LDL and triglycerides. Administration of amla extract orally in the dosage of 10 and 20 mg/kg produced significant antihypercholesterolaemic effect in these experimental animals. The rabbits of untreated hypercholesterolaemic control group did not show marked decrease in serum cholesterol, LDL, VLDL and triglyceride levels, while animals of treatment group showed significant decrease (P < 0.05) at the end of the first month of treatment. The results were in agreement with earlier studies conducted using amla fruit/fresh amla juice/amla extract in animals [2-5].
There are no earlier published reports on the effect of amla extract on the HDL-C levels. The results of this study show significant (P < 0.05) elevation in the HDL cholesterol levels in the treatment groups after 3 mo of oral feeding with amla extract. The cholesterol levels of tissues such as liver, heart and kidney were decreased significantly (P < 0.05) in the treatment groups.
The activity of enzyme HMG CoA reductase was lower in the treatment group compared to untreated hypercholesterolaemic control group or normal group of rabbits. This was in accordance with studies conducted by Anila6. This indicates that feeding of amla extract decreases the synthesis of cholesterol by inhibiting HMG CoA reductase activity.
Treatment with amla extract showed significant decrease in LDL levels, which may be a reason for the reversal or absence of atherosclerosis in groups C and D. amla extract, by its antioxidant action, prevents LDL oxidation, thereby inhibiting endothelial dysfunction and formation of atheromatous plaques.
In conclusion, our results have shown that treatment with amla extract can reverse the atheromatous plaque formation in the blood vessels of rabbits due to a number of factors like preventing the initiation of early endothelial dysfunction by its antioxidant action, preventing LDL oxidation, decreasing LDL cholesterol. HMG CoA reductase inhibition results in decreased synthesis of cholesterol. Increase in HDL enhances the reverse transport of circulating cholesterol. By a combination of these effects, amla extract can effectively prevent further extension of atheromatous plaques and promote reversal of already formed lesions.
As amla extract is a natural product made from fresh amla fruit, which is commonly used as a traditional Ayurvedic drug without any reported side effects, it can be used as an effective antidyslipidaemic and antiatherogenic agent. Many studies have come to the conclusion that with a 1% decrease in total cholesterol levels, the incidence of coronary artery disease (CAD) decreases by 2% [19]. There was a 10% increase in CAD for each 4 mg/dl decrease in HDL [20]. Prospective studies evaluated the effects of raising the low HDL cholesterol levels in people with and without a history of CAD [21,22]. The amla extract used in this study was the product developed by the Research and Development laboratory of M/s Arjuna Natural Extracts Ltd.
Acknowledgements
The authors deeply acknowledge the valuable suggestions and help received from Dr. Santhakumary (Retd. DME) and Dr. S. B. Rao while carrying out this work.
References
- Satyavati, G.V., Raina, M.K. and Sharma, M., In; Medicinal Plants ofIndia, Vol. I, Indian Council of Medical Research, New Delhi,1976, 377
- Mishra, M., Pathak, U.N. and Khan, A.B., Brit. J. Exp. Pathol.,1981, 62, 526.
- Mathur, R., Sharma, A., Dixit, V.P. and Varma, M., J.Ethnopharmacol.,1996, 50, 261.
- Thakur, C.P. and Mandal, K., Indian J. Med. Res., 1984, 79, 142.
- Annu, J., Pandey, M., Kapoor, S. and Saroja, R., Eur. J. Clin.Nutr., 1988, 42, 939.
- Anila, L. and Vijayalakshmi, N.R., J. Ethnopharmacol., 2002, 79, 81.
- Bhattacharya, S.K., Bhattacharya, A., Sairam, K. and Ghosal, S., Phytomedicine, 2002, 9, 171.
- Tariq, M., Hussain, S.J., Asif, M. and Jahan, M., Indian J. Exp.Biol., 1977, 15, 485.
- Roeschlau, P., In; Bergmeyer, H.U., Eds., Methods of EnzymaticAnalysis, 2nd Edn., Academic Press, New York, 1974, 1980.
- Burstein, M., J. Lipid Res., 1970, 19, 583.
- Bucolo, G., Clin. Chem., 1973, 19, 476.
- Frieldwald, W.T., Levi, R.I. and Friedericksn, D.S., Clin. Chem.,1972, 18, 499.
- Luna, L.G., Eds., In; Manual of histology, 3rd Edn., New York, McGraw Hill, 1968.
- Radin, N., In; Lowenstein, J.M., Eds., Methods in Enzymology,Academic Press, New York, 1981, 72, 5.
- Valsa, A.K., Ushakumari, B. and Vijayalakshmi, N.R., J .Clin.Biochem. &Nutri.,1995, 19, 175.
- Sheela, C.G. and Augusti, K.T., Indian J. Exp. Biol. 1975, 33, 337.
- Gorden, D. and Rifkind, H.M., New Engl. J. Med., 1989, 321, 1311.
- Brewer, Jr., H.B., N. Engl. J. Med., 2004, 350, 1491.
- Levini, G.N. and Keaney, J.F., Jr. Vita, J.A., N. Engl. J. Med., 1995,332, 512.
- Castelli, W.P., Garrison, R.J. and Wilson, P.W., J. Amer. Med.Assoc., 1986, 256, 2835.
- Rubins, H.B., Robins, S.J. and Collins, D., N. Engl. J. Med., 1999,341,410.
- Downs, J.R., Clearfield, M., Weis, S., Whipney, E., Shapiro, D.R. and Beere, P., J. Amer. Med. Assoc., 1998, 279,1615.