- *Corresponding Author:
- F. Wang
Department of Anesthesiology, Hengshui People's Hospital, Hengshui, Hebei Province 053000, China
E-mail: 13831869389@163.com
| Date of Received | 27 November 2021 |
| Date of Revision | 04 December 2022 |
| Date of Acceptance | 06 July 2023 |
| Indian J Pharm Sci 2023;85(4):979-986 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of sevoflurane combined with ulinastatin on cell proliferation and migration of human lung adenocarcinoma. Human lung adenocarcinoma cells H1975 were cultured in vitro, and treated with different concentrations of sevoflurane, ulinastatin and sevoflurane+ulinastatin, the cells were divided into control group, sevoflurane, ulinastatin and sevoflurane+ulinastatin group by cell counting kit 8; H1975 cell cycle by flow cytometry; the number of H1975 cells was measured by Transwell; protein immunoblot (Western blot). Compared with the control group, different concentrations of sevoflurane and ulinastatin treatment significantly inhibited the proliferation of H1975 cells in a dose-dependent manner. Sevoflurane combined with ulinastatin had a synergistic effect on cell proliferation inhibition in H1975 cells. Compared with the control group, the inhibition of cell proliferation and the proportion of cells in growth 0/growth 1 phase were significantly increased in the sevoflurane and ulinastatin groups, the proportion of cells in S phase, the number of cell migration, proliferation proteins cyclin D1, cyclin-dependent kinase 4, migration protein matrix metalloproteinase-2 and matrix metalloproteinase-9 were significantly decreased (p<0.05); compared with the sevoflurane group and the ulinastatin group, the inhibition of cell proliferation and the percentage of cells in growth 0/growth 1 group, the proportion of cells in S phase, cell migration number, proliferation proteins cyclin D1, cyclin-dependent kinase 4, migration proteins matrix metalloproteinase-2 and matrix metalloproteinase-9 were significantly decreased (p<0.05). Sevoflurane combined with ulinastatin can synergistically downregulate proliferation and migration protein expression and then inhibit the proliferation and migration of lung adenocarcinoma cells H1975, which may be a potential treatment for lung adenocarcinoma in clinical practice.
Keywords
Sevoflurane, ulinastatin, lung adenocarcinoma, proliferation, migration
Lung cancer is one of the malignant tumors that affect human health. With the economic development and environmental deterioration, the mortality rate of lung cancer gradually increases[1]. Lung adenocarcinoma is a common type of lung cancer, accounting for about 40 %-50 % of lung cancer. Surgery is one of the main means of lung cancer treatment. However, after surgical treatment and adjuvant chemotherapy, some patients will still relapse or have distant metastasis, which is the main cause of death[2,3]. Inhibition of lung cancer metastasis is the key to improve the prognosis and survival rate of lung cancer patients clinically. Surgical stress and the use of anesthetic are able to affect the proliferation, migration and invasion of tumor cells[4,5]. Sevoflurane is a commonly used general anesthetic drug during surgery and studies have shown that it can inhibit the proliferation of lung cancer, colon cancer, breast cancer and other cancer cells[6-8]. Ulinastatin is a widely used drug in the operating room and intensive care unit, which has been proven to inhibit tumor progression in lung cancer, gliomas[9,10] and has an important role in antitumor therapy. Previous studies found that the combination of sevoflurane and ulinastatin improved the inflammation and ischemic injury caused by surgery[11,12], However, there are no studies on the effect of sevoflurane combined with ulinastatin in anti-tumor. In this study, lung adenocarcinoma cells were cultured and treated in vitro to investigate the effect of the combination on the proliferation and migration, which provided a theoretical basis for the use of sevoflurane and ulinastatin in clinical lung cancer surgery.
Materials and Methods
Drugs and reagents:
The human lung adenocarcinoma cell, NCI-H1975 (KCB20140342YJ), the Kunming Cell Bank of the Chinese Academy of Sciences; sevoflurane (H20173007), Shanghai Hengrui Pharmaceutical Co., Ltd.; ulinastatin (Chinese medicine approved H19990134), Guangdong Tianpu Biochemical Pharmaceutical Co., Ltd.; Fetal Bovine Serum (FBS) (10099), penicillin-streptomycin (15070063), Roswell Park Memorial Institute (RPMI)-1640 medium (31800022), trypsin (25200056), Gibco, Inc.; cyclin D1 (cyclin D1) (55,506), cyclin-dependent kinase, or Cyclin-Dependent Kinase 4 (CDK 4) (12790), Matrix Metalloproteinase (MMP)-2 (40994), MMP 9 (13667), Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (5174) primary antibodies, American cell signaling technology company; goat anti-rabbit Immunoglobulin G IgG (H+L) (ab205718), UK Abcam company; protein extraction kit (P0033), Bicinchoninic Acid (BCA) protein quantification kit (P0012), Cell Counting Kit- 8 (CCK-8) (C0037), cell cycle detection kit (C1052), Shanghai Biyuntian Biotechnology Co., Ltd.
Multifunction Microplate Reader (Varioskan™ LUX), Thermo Fisher, United States of America (USA); flow cytometry (FACS Canto II); protein gel imager (Gel Doc), Bio-Rad, USA.
Experimental method:
Cell culture: Human lung adenocarcinoma cells H1975 were routinely revived to RPMI-1640 medium supplemented with 10 % fetal calf serum, 100 U/ml penicillin-streptomycin at 37°, 5 % Carbon dioxide (CO2) cultures in the incubator and cells growing stably to log phase were used for subsequent experiments.
CCK-8 assay: CCK-8 assay was used to test the effect of sevoflurane combined with ulinastatin on H1975 cell proliferation. H1975 cells were divided into control groups, adding fresh medium only; sevoflurane group, cells were placed in sterile closed containers containing different concentrations (2 %, 4 %, 6 %, 8 %, 7 % and 10 %) and ulinastatin group, adding medium containing different concentrations (100, 200, 400, 600 and 800 U/ml).
Growth to log phase H1975 cells were collected in cell culture, trypsin digested and the cell concentration adjusted to 5×103 individual/well added to 96-well plates as per reference methods, five 96-well plates were placed in sterile closed containers[13], with inlet and outlet connections. The anesthesia machine connects with the air inlet and a sevoflurane vaporizer to supply the sevoflurane gas (95 % Oxygen (O2) to the container 2/5 % CO2 mixing). The concentration of sevoflurane in the container is monitored by a gas monitor, which is connected to the outlet port of the container. Sevoflurane (2 %, 4 %, 6 %, 8 %, 10 %) to 5 % CO2, 95 % O2 H1975 cells mixed with the gas were control cells and after 6 h, 96-well plates were transferred to the incubator and incubated for a further 48 h. Then, six complex wells were selected for each group, 10 μl CCK-8 reagent was added, 37° was protected from light for 2 h, and the supernatant was discarded. The Optical Density (OD) value of absorbance per well was detected at the wavelength of 450 nm in the microplate reader.
Growth to log phase H1975 cells were collected in cell culture, trypsin digested and the cell concentration adjusted to 5×103 individual/wells to 96-well plates, add different concentrations (100, 200, 400, 600 and 800 U/ml), control group add only fresh medium, continue for 48 h, set 6 wells for each group, add 10 μl CCK-8 reagent, cultivate 37° from light for 2 h, discard the supernatant, test the absorbance OD of each well at 450 nm in the microplate reader.
Inhibition rate of cell proliferation=(Experimental OD/control OD)×100 %
Sevoflurane+ulinastatin group collected growth to 1 log phase H1975 cells in cell culture, trypsin digested, adjust cell concentration to 5×103 and added to 96-well plates, the control group added 100 μl of fresh medium per well having 5 % CO2, 95 % O2 in a sterile closed container with the gas mixture, the sevoflurane+ulinastatin group was placed into an sterile closed container containing 2 % sevoflurane (no inhibition to cells), and added ulinastatin medium containing different concentrations (100, 150, 200, 250 and 300 U/ml) for 48 h, after culture, 6 complex wells were set in each group, 10 μl CCK-8 reagent was added, 37° light culture for 2 h, discard the supernatant, test the absorbance OD value of each well at 450 nm in the microplate reader to detect the inhibition of cell proliferation. CompuSyn software calculates half-maximal Inhibitory Concentration (IC50), Combination Index (CI), if CI<1 means synergy, CI=1 means additive and CI>1 means antagonism.
Cell cycle detection of H1975 by flow cytometry: According to the combination results of sevoflurane and ulinastatin in CCK-8 assay, the minimum CI of H1975 cells were treated with sevoflurane and ulinastatin. H1975 cells were divided into control group (adding fresh medium only), sevoflurane group (adding sevoflurane containing concentration of 50 μmol/l), ulinastatin group (adding ulinastatin medium containing concentration of 200 U/ml), and sevoflurane+ulinastatin group (adding medium containing 50 μmol/l sevoflurane and 200 U/ml ulinastatin).
Log phase cells from each group were collected and trypsin digested and cell concentration adjusted to 1×106 l/ml, 100 μl of cell suspension was absorbed and inoculated into 6-well plates, and each group was set in triplicate. After 48 h of culture, cells were collected, washed in Phosphate Buffered Saline (PBS), fixed in 75 % ethanol on ice for 30 min, washed with PBS, added Propidium Iodide (PI) and Ribonuclease A (RNase A) and incubated for 30 min. Deoxyribonucleic Acid (DNA) content in each period was observed by flow cytometry.
Cell migration capacity was determined by Transwell assay: Logarithmic phase H1975 cells in cell cycle were removed for trypsin digestion and adjusted the cell concentration to 5×105 individual/ ml. 100 μl of cell suspension was absorbed to the upper chamber of the Transwell chamber, 600 μl of RPMI-1640 medium containing FBS was added to the Transwell chamber, cultured for 24 h, inverted into the chamber medium, unmigrated cells were gently wiped away with a cotton swab, fixed with anhydrous methanol, stained with 0.1 % crystal violet solution and the stained cells were counted from five randomly selected fields under the microscope.
Protein proliferation and migration protein expression was detected by immunoblot (H1B): Take H1975 cells from each group in cell cycle, perform trypsin digestion and adjust the cell concentration to 1×106 individual/well and were added to 24-well plates, and cultured for 24 h, supernatant was discarded, washed with PBS, total cellular protein was extracted with protein extraction kit and total protein concentration was determined by BCA kit. Protein samples were subjected to Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), membrane transfer and blocked; primary antibody dilution (cyclin D1, CDK 4, MMP 2, MMP 9, GAPDH 1:1000), incubated at 4° overnight, washed with Tris-Buffered Saline with 0.1 % Tween® 20 (TBST), goat anti-rabbit IgG (H+L) (1:2000), incubated for 1 h and washed with TBST. Protein gel imaging and protein expression levels were analyzed by ImageJ software.
Statistical methods:
Statistical analysis of the data was performed using Statistical Package for the Social Sciences (SPSS) 25.0. Geometric data were described by mean±standard deviation (x̄ ±s), one-way Analysis of Variance (ANOVA) for means between multiple groups and pair-wise comparisons by Student– Newman–Keuls-Q (SNK-Q) method, with p<0.05 considered statistically significant.
Results and Discussion
Compared with the control group, the sevoflurane group gradually increased cell proliferation inhibition and showed dose-dependent (p<0.05), and the IC50 of sevoflurane on H1975 cells was 6.03 % and 10 μmol/l was 1.95%; with sevoflurane, concentrations were performed for subsequent experiments as shown in Table 1.
| Group | Concentration (μmol/l) | Cell proliferation inhibition rate (%) |
|---|---|---|
| Control | - | 0.00 |
| Sevoflurane | 2 % | 11.73±2.12a |
| 4 % | 32.45±2.85a | |
| 6 % | 43.72±2.67a | |
| 8 % | 62.81±3.14a | |
| 10 % | 75.64±2.93a |
Note: Compared with the control group, ap<0.05
Table 1: Effects of Sevoflurane on Cell Proliferation of H1975 (n=6, x̄±s)
Compared with the control group, the inhibition of cell proliferation in the ulinastatin group gradually increased and appeared dose-dependent (p<0.05), and the IC50 of ulinastatin on H1975 cells was 496.45 U/ml as shown in Table 2.
| Group | Concentration (U/ml) | Cell proliferation inhibition rate (%) |
|---|---|---|
| Control | 0.00 | |
| Ulinastatin | 100 | 8.45±1.96a |
| 200 | 16.17±2.25a | |
| 400 | 33.52±2.67a | |
| 600 | 57.39±2.94a | |
| 800 | 75.82±2.79a |
Note: Compared with the control group, ap<0.05
Table 2: Effects of Ulinastatin on the Proliferation of H1975 CELLS (n=6, x̄±s)
To give an IC without inhibition of the cells 102 % sevoflurane combined with different concentrations of ulinastatin (not more than the IC50 of ulinastatin) acting on H1975 cells. 2 % sevoflurane at different concentrations (100, 200, 300 and 400), with CI<1. At 2 % sevoflurane+200 U/ml ulinastatin, this combined concentration was selected for subsequent experiments as shown in Table 3.
| Sevoflurane | Ulinastatin (U/ml) | Cell proliferation inhibition rate (%) | CI |
|---|---|---|---|
| 2 % | 100 | 46.32±3.41 | 0.580±0.08 |
| 200 | 62.54±5.64 | 0.552±0.06 | |
| 300 | 66.77±4.25 | 0.631±0.07 | |
| 400 | 73.48±3.96 | 0.627±0.09 |
Table 3: Inhibition and CI of H1975 Cell Proliferation by Sevoflurane Combined with Ulinastatin (n=6, x̄±s)
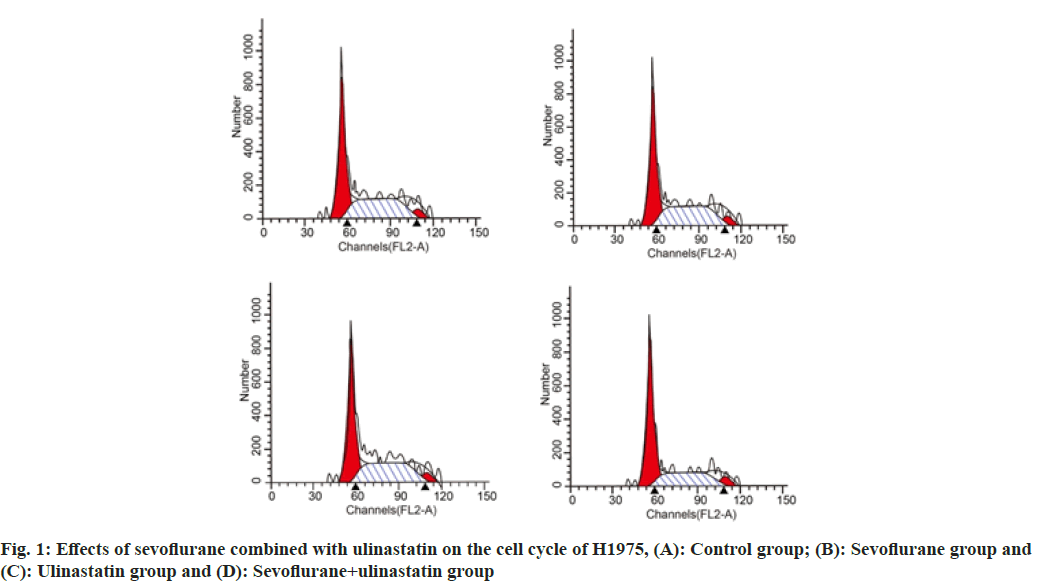
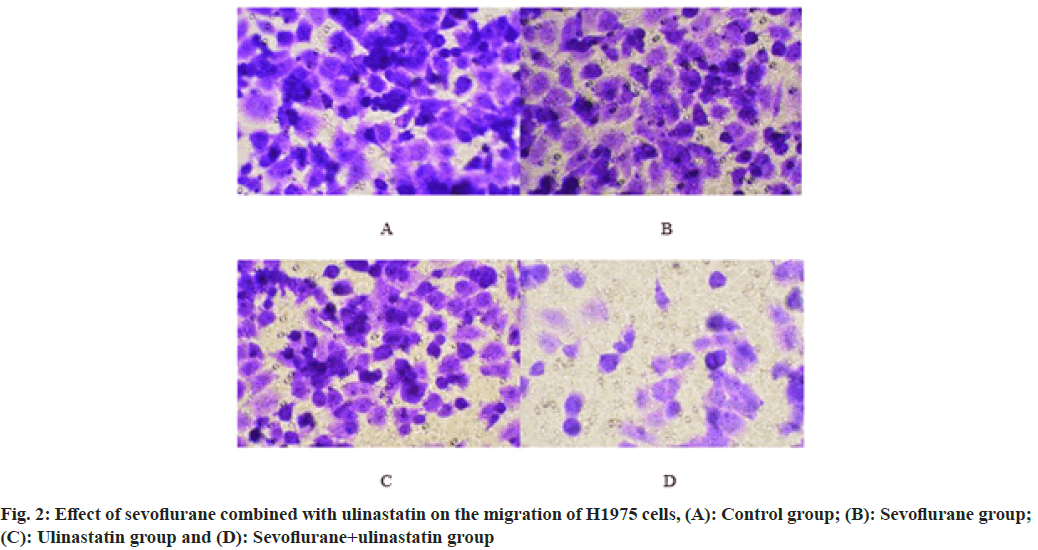
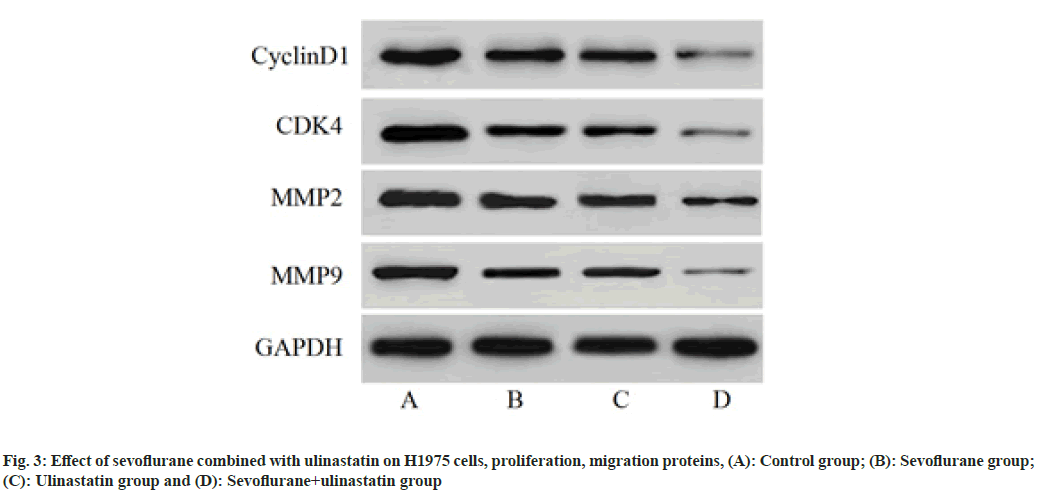
Compared with controls, the proportion of cells in G0/G1 was significantly increased (p<0.05), and cells in S phase (p<0.05) as shown in Table 4 and fig. 1. The number of cells migrated in sevoflurane, ulinastatin and sevoflurane+ulinastatin (p<0.05), were shown in Table 5 and fig. 2. Cyclin D1, CDK 4, MMP 9 (p<0.05), MMP 2, MMP 9 were significantly decreased in the group (p<0.05) as shown in Table 6 and fig. 3.
| Group | Percentage of cells (%) | ||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Control | 43.12±1.29 | 37.71±1.35 | 18.35±1.25 |
| Sevoflurane | 52.47±1.36a | 30.45±1.17a | 17.63±0.94 |
| Ulinastatin | 56.36±1.41a | 62.67±1.22a | 17.25±0.78 |
| Sevoflurane+ulinastatin | 65.48±1.27abc | 19.28±1.34abc | 16.41±0.86 |
Note: Compared with the control group, ap<0.05; when compared with the sevoflurane group, bp<0.05 and compared with the ulinastatin group, cp<0.05
Table 4: Effect of Sevoflurane and Ulinastatin on the Cell Cycle of H1975 (n=6, x̄±s)
| Group | Number of cell migration (s) |
|---|---|
| Control | 138.46±13.52 |
| Sevoflurane | 98.35±10.29a |
| Ulinastatin | 79.21±9.34a |
| Sevoflurane+ulinastatin | 51.34±6.42abc |
Note: Compared with the control group, ap<0.05; when compared with the sevoflurane group, bp<0.05 and compared with the ulinastatin group, cp<0.05
Table 5: Effect of Sevoflurane Combined with Ulinastatin on the Migration of H1975 Cells (n=3, x̄±s)
| Group | Cyclin D1/GAPDH | CDK4/GAPDH | MMP2/GAPDH | MMP9/GAPDH |
|---|---|---|---|---|
| Control | 1.12±0.08 | 1.17±0.07 | 0.95±0.08 | 0.89±0.05 |
| Sevoflurane | 0.83±0.06a | 0.75±0.05a | 0.61±0.04a | 0.58±0.03a |
| Ulinastatin | 0.74±0.05a | 0.69±0.07a | 0.57±0.05a | 0.46±0.05a |
| Sevoflurane+ulinastatin | 0.25±0.04abc | 0.19±0.06abc | 0.12±0.06abc | 0.09±0.04abc |
Note: Compared with the control group, ap<0.05; when compared with the sevoflurane group, bp<0.05 and compared with the ulinastatin group, cp<0.05
Table 6: Effects of Sevoflurane and Ulinastatin on H1975 Cells (n=3, x̄±s)
Surgical resection is the main treatment for primary tumors, however surgery may increase the entry of malignant cells into the blood and lymphatic circulation, inhibit their apoptosis and enhance their metastatic capacity[14]. The use of anesthetics is considered to be one of the main factors affecting the recurrence and metastasis of malignancy, with different anesthetic influences on cancer cell proliferation and migration, and the prognosis of cancer patients[15]. Studies show that intraoperative opioid use is associated with reduced overall survival in patients with non-small cell lung cancer[16]. Chu et al. studies have shown that the anesthetic etomidate can inhibit the proliferation and migration of lung adenocarcinoma cells A549[17]. The proliferation and migration of tumor cells are the basis of tumor metastasis and anesthetic drugs may affect the prognosis of patients undergoing cancer surgery by affecting their migration and invasion, and then affecting the recurrence and metastasis of cancer. Therefore, in this study, the effects of sevoflurane and ulinastatin on lung adenocarcinoma cell proliferation and migration were investigated by growing lung adenocarcinoma cells H1975 in vitro and treated with different concentrations of sevoflurane, ulinastatin and sevoflurane+ulinastatin.
Sevoflurane is a colorless, transparent and nonirritating volatile anesthetic that has been widely used in the surgical process of malignant tumors, including lung cancer. Studies have shown that sevoflurane can inhibit the proliferation and migration of various tumor cells, such as Chen et al.[18] studies have shown that sevoflurane is able to inhibit the proliferation and invasion of osteosarcoma cells. Some studies have shown that 3 % sevoflurane treatment can promote the apoptosis of A549 in lung cancer cells, which may reduce the cancer cell spread caused by surgery. Liang et al.[19] sevoflurane inhibited platelet-induced invasion of lung cancer cells by reducing platelet activity. Ulinastatin is a widespread protease inhibitor found in nature. Application in surgery cannot only improve the general condition of patients, but also improve the immune function decline caused by surgical stimulation, and alleviate the organ and cell damage caused by surgery[20]. Zhou et al.[21] studies have shown that lung complications in patients treated with ulinastatin have a lower frequency than those in the control group. With the application of ulinastatin in surgery, it was found to inhibit the proliferation and migration of tumor cells. Wang et al.[22] studies have shown that ulinastatin can inhibit the proliferation, invasion and migration of SGC-7901 in gastric cancer cells. The results of this study showed that sevoflurane and ulinastatin treatment alone could inhibit the proliferation of lung adenocarcinoma cells H1975, and the inhibition rate of cell proliferation gradually increased with the increase of use concentration, suggesting that both may have a certain role in the inhibition of metastasis in lung cancer.
Inter drug synergy is able to promote drug inhibition of cancer cell proliferation, migration and increase drug sensitivity, Hu et al.[23] studies have shown that ulinastatin can inhibit the stemness of hepatoma cells and enhance its 5-fluorouracil sensitivity, and can be used as a chemical sensiensitizer of 5-fluorouracil. Li et al.[24] studies have shown that ulinastatin and protocol synergistically inhibit the proliferation and migration of human lung adenocarcinoma cells A549 and promote cell apoptosis. The results of this study showed that sevoflurane and ulinastatin had a synergistic effect on proliferation inhibition of H1975 cells, and the proliferation and migration of H1975 were significantly higher than that of sevoflurane and ulinastatin alone, suggesting that the administration of sevoflurane and ulinastatin during surgery for lung adenocarcinoma. Cancer metastasis, or the spread of tumors from the primary site to distant organs, is a complex process associated with the proliferation and migration of cancer cells[25]. This study found that compared with sevoflurane group and ulinastatin group, the proportion of cells in G0/G1 phase was significantly increased in sevoflurane group and ulinastatin group, and the proportion of cells and cell migration in S phase were significantly decreased. Protein cyclin D1 and CDK4 are proliferative proteins related to the cell cycle. MMP can degrade various protein components in the cytoplasmic matrix. They are important structural molecules controlling the adhesion and invasion of cancer cells and are related to the migration of cancer cells[26]. The experiment further showed that sevoflurane combined with ulinastatin group could further inhibit the expression of proteins cyclin D1, CDK4, MMP 2 and MMP 9, suggesting that the application of sevoflurane combined with ulinastatin in clinical surgery may help to reduce the recurrence and metastasis of lung adenocarcinoma. The specific mechanism needs further study.
In conclusion, sevoflurane and ulinastatin have synergistic effects on the proliferation, migration and inhibition of lung adenocarcinoma cells H1975, and their inhibitory effect is related to the downregulation of proliferation and migration protein expression, which may be a potential drug collocation use regimen in clinical surgery. However, this study only investigated the effects of sevoflurane and ulinastatin on one type of lung adenocarcinoma cells. The next step is to study the combination role in other lung cancer cells, and to study the relevant mechanisms, so as to provide a theoretical basis for the choice of anesthetic in lung cancer surgery.
Funding:
This work was supported by application of anterior paravertebral nerve block under direct vision in thoracotomy surgery (No: 2018014023Z).
Conflict of interests:
The authors declared no conflict of interests.
References
- Skrickova J, Kadlec B, Venclicek O, Merta Z. Lung cancer. Cas Lek Cesk 2018;157(5):226-36.
[PubMed]
- Zhang M, Li C, Li G. The influence of pre-metastatic microenvironment regulation on lung cancer metastasis and the current status of its clinical application. Chin J Pharm 2020;55(11):871-4.
- Zhang M, Deng H, Li Y. The LHX6 gene suppresses the metastasis mechanism in lung adenocarcinoma. J Pract Med 2019;35(22):3447-51.
- Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis 2018;35(4):347-58.
[Crossref] [Google Scholar] [PubMed]
- Zheng X, Dong L, Zhao S, Li Q, Liu D, Zhu X, et al. Propofol affects non–small-cell lung cancer cell biology by regulating the miR-21/PTEN/AKT pathway in vitro and in vivo. Anesth Analg 2020;131(4):1270-80.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Wang T, Gu JQ, Su HB. Volatile anesthetic sevoflurane suppresses lung cancer cells and miRNA interference in lung cancer cells. Oncol Targets Ther 2018;11:5689-93.
[Crossref] [Google Scholar] [PubMed]
- He J, Zhao H, Liu X, Wang D, Wang Y, Ai Y, et al. Sevoflurane suppresses cell viability and invasion, and promotes cell apoptosis in colon cancer by modulating exosome-mediated circ-HMGCS1 via the miR-34a-5p/SGPP1 axis. Oncol Rep 2020;44(6):2429-42.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Yang L, Guo X, Jin G, Wang Q, Lv D, et al. Sevoflurane suppresses proliferation by upregulating microRNA-203 in breast cancer cells. Mol Med Rep 2018;18(1):455-60.
[Crossref] [Google Scholar] [PubMed]
- Song B, Bian Q, Shao CH, Li G, Liu AA, Jing W, et al. Ulinastatin reduces the resistance of liver cancer cells to epirubicin by inhibiting autophagy. PLoS One 2015;10(3):e0120694.
- Lin D, Zhu X, Li J, Yao Y, Guo M, Xu H. Ulinastatin alleviates mitochondrial damage and cell apoptosis induced by isoflurane in human neuroglioma H4 cells. Hum Exp Toxicol 2020;39(10):1417-25.
[Crossref] [Google Scholar] [PubMed]
- Zhang J. Clinical study of the effect of sevoflurane compound ulinastatin on patients with craniotomy aneurysms. Central South Univ J 2011;9(17):17-29.
- Nishiyama T, Yokoyama T, Yamashita K. Effects of a protease inhibitor, ulinastatin on coagulation and fibrinolysis in abdominal surgery. J Anesth 2006;20(3):179-82.
[Crossref] [Google Scholar] [PubMed]
- Kang K, Wang Y. Sevoflurane inhibits proliferation and invasion of human ovarian cancer cells by regulating JNK and p38 MAPK signaling pathway. Drug Des Devel Ther 2019;13(1):4451-60.
[Crossref] [Google Scholar] [PubMed]
- Yang W, Cai J, Zabkiewicz C, Zhang H, Ruge F, Jiang WG. The effects of anesthetics on recurrence and metastasis of cancer, and clinical implications. World J Oncol 2017;8(3):63-70.
[Crossref] [Google Scholar] [PubMed]
- Fan X, Wang D, Chen X, Wang R. Effects of anesthesia on postoperative recurrence and metastasis of malignant tumors. Cancer Manag Res 2020;12:7619-33.
- Cata JP, Keerty V, Keerty D, Feng L, Norman PH, Gottumukkala V, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med 2014;3(4):900-8.
[Crossref] [Google Scholar] [PubMed]
- Chu CN, Wu KC, Chung WS, Zheng LC, Juan TK, Hsiao YT, et al. Etomidate suppresses invasion and migration of human A549 lung adenocarcinoma cells. Anticancer Res 2019;39(1):215-23.
[Crossref] [Google Scholar] [PubMed]
- Chen M, Zhou L, Liao Z, Ye X, Xuan X, Gu B, et al. Sevoflurane inhibited osteosarcoma cell proliferation and invasion via targeting miR-203/WNT2B/Wnt/β-catenin axis. Cancer Manag Res 2019;11:9505-15.
[Crossref] [Google Scholar] [PubMed]
- Liang H, Yang CX, Zhang B, Zhao ZL, Zhong JY, Wen XJ. Sevoflurane attenuates platelets activation of patients undergoing lung cancer surgery and suppresses platelets-induced invasion of lung cancer cells. J Clin Anesth 2016;35:304-12.
[Crossref] [Google Scholar] [PubMed]
- Lagoo JY, D'Souza MC, Kartha A, Kutappa AM. Role of ulinastatin, a trypsin inhibitor, in severe acute pancreatitis in critical care setting: A retrospective analysis. J Crit Care 2018;45:27-32.
[Crossref] [Google Scholar] [PubMed]
- Zhou L, Lan H, Zhou Q, Tang XJ, Zhu D, Yue J, et al. Continuous infusion of high-dose ulinastatin during surgery does not improve early postoperative clinical outcomes in patients undergoing radical lung cancer surgery: A pilot study. Thorac Cancer 2016;7(5):581-7.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Chen X, Su L, Zhu Z, Wu W, Zhou Y. Suppressive effects on cell proliferation and motility in gastric cancer SGC-7901 cells by introducing ulinastatin in vitro. Anticancer Drugs 2016;27(7):651-9.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Ding J, Wang G, Zhang X. The combination of ulinastatin and 5-fluorouracil synergistically inhibits hepatocellular carcinoma growth. J Int Med Res 2020;48(3):3.
[Crossref] [Google Scholar] [PubMed]
- Li P, Guo P, Lin C, He M, Zhu X, Liu C, et al. The synergistic effect of propofol and ulinastatin suppressed the viability of the human lung adenocarcinoma epithelial A549 cell line. Oncol Lett 2018;16(4):5191-9.
[Crossref] [Google Scholar] [PubMed]
- Janssen LM, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: Friend or foe? J Immunother Cancer 2017;5(1):79.
[Crossref] [Google Scholar] [PubMed]
- Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 2017;147:1-73.
[Crossref] [Google Scholar] [PubMed]