- *Corresponding Author:
- MA JS
Function Experiment Teaching Center, Wenzhou Medical College, Wenzhou?325 035, China
E-mail: jianshe160@yahoo.com.cn
| Date of Received : | 01 March 2012 |
| Date of Revised : | 04 January 2013 |
| Date of Accepted : | 08 January 2013 |
| Indian J Pharm Sci 2013;75(1):94-98 |
Abstract
Wuniu early tea (Camellia sinensis) is an important beverage consumed in China. Up to date, a lot of methods for identifying and chemical analysing have been done. However, there is no report on the effects of Wuniu early tea on cytochrome P450 isozymes. Therefore, the present objective of our study was to evaluate the potential effects of Wuniu early tea on cytochrome P450 isozymes P2C9, P1A2, P2C19 and P2B6 in rats with a cocktail approach including, matching probe drugs of tolbutamide, phenacetin, omeprazole and bupropion. These four probe drugs were simultaneously administered to rats after repeated Wuniu early tea administration. The pharmacokinetics of the probes in the plasma was simultaneous determined by high-performance liquid chromatography-mass spectrometry. The t 1/2 and AUC (0-∞) of tolbutamide increased significantly and CL z decreased remarkably in test rats after repeated Wuniu early tea administration. However, the main pharmacokinetic parameters of the other three probe drugs were not significantly different between control and test rats. The findings in this study suggested that Wuniu early tea could inhibit cytochrome P2C9 while did not influence on cytochrome P1A2, cytochrome P2C19 and cytochrome P2B6.
Keywords
Cytochrome P450, cocktail method, Wuniu early tea
Wuniu early tea (Camellia sinensis), originated in Wuniu town, Yongjia county of Zhejiang province in China, is one of the most consumed beverages in the world. It has been reported to have anticancer, antibiosis, antiaging and antiinflammatory properties and promote weight loss. The main active constituent is believed to be catechins, which account for 26.6% in Wuniu early tea. There is a significant signal that foods, beverages and dietary supplements containing high levels of phytochemicals may participate in clinically significant deleterious interactions with traditional prescription and over‑the‑counter drugs [1‑3]. The wide consumption of Wuniu early tea in the general public suggests that the possibility for coadministration with synthetic drugs and accordingly, the potential for drug interactions are high. Till today, various studies on Wuniu early tea pharmacological actions have been done. However, the inhibitive and inducing effects of Wuniu early tea on the activity of the cytochrome P450 (CYP450) enzymes involved in drug metabolism are unknown.
CYP450 is major phase I metabolic enzyme, widely expressed in major body organs and play an important role in the biotransformation of almost all medications. Specific probe drugs have been widely used for assessing various individual CYP450 enzymes activities [4]. So‑called cocktail methods, in which several enzyme activities are determined simultaneously, after the concurrent administration of many CYP‑specific probes, have been described and developed in recent years [5‑10]. In addition, the cocktail approach involving multiple probe drugs have been widely used for predicting the effect of a new chemical entity on the CYP450 activities and the potential drug–drug interactions [11‑17].
To our knowledge, this is the first study assessing whether repeated Wuniu early tea administration may participate in drug interactions by affecting the CYP450 enzyme activity. We used a drug cocktail strategy consisting of tolbutamide, phenacetin, omeprazole, bupropion respectively to investigate the effects of Wuniu early tea on CYP450 activities including CYP2C9, CYP1A2, CYP2C19 and CYP2B6 in rats.
Tolbutamide, phenacetin, omeprazole, bupropion and the internal standard (IS) carbamazepine were purchased from Sigma‑Aldrich Company (St. Louis, USA). High‑performance liquid chromatography (HPLC) grade acetonitrile and methanol were from Merck Company (Darmstadt, Germany). All other chemicals were of analytical grade. Ultrapure water (resistance >18 mΩ) prepared by a Millipore Milli‑Q purification system (Bedford, USA).
Wistar rats with body weights of 180±30 g were provided by the Animal Care and Use Committee of Wenzhou Medical College. They were housed into house cages at 20‑24° and allowed free access to regular rodent diet and water.
Twelve male Wistar rats (180±30 g) were randomly divided into two groups, control group and test group. The ratio of Wuniu early tea with water was 1:100 and draw for 15 min. Wuniu early tea was administered as drinking water for test group and water was given in the same way for control group. After 7‑day oral administration of Wuniu early tea, rats of the two groups were administered by gastric irrigation with the dose of 3, 15, 15 and 15 mg/kg for tolbutamide, phenacetin, omeprazole and bupropion, respectively.
Blood samples 0.3 ml were obtained through the tail vein into heparinized 1.5 ml polythene tubes at 0.083, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 12 and 24 h after the administration of probe drugs. The samples were immediately centrifuged at 5000 rpm for 10 min, 100 μl plasma were transferred to another tube and stored at −20° until analysis.
All analysis was performed with a 1200 Series Liquid Chromatograph (Agilent Technologies, Waldbronn, Germany) equipped with a quaternary pump, a degasser, an auto sampler, a thermostatted column compartment, and a Bruker Esquire HCT mass spectrometer (Bruker Technologies, Bremen, Germany) equipped with an electrospray ion source and controlled by ChemStation software.
Chromatographic separation was achieved on a 150×2.1 mm, 3.5 μm particle, Agilent Zorbax SB‑C18 column at 30°. A gradient elution programme was conducted for chromatographic separation with mobile phase A (0.1% formic acid in water) and mobile phase B (acetonitrile) as follows: 0‑1.5 min (10‑85% B), 1.5‑6.0 min (85‑85% B), 6.0‑7.0 min (85‑10% B), 7.0‑10.0 min (10‑10% B). The flow rate was 0.4 ml/ min.
The quantification was performed by the peak‑area method. The determination of target ions were performed in SIM mode (m/z 271 for tolbutamide, m/z 180 for phenacetin, m/z 346 for omeprazole, m/z 240 for bupropion and m/z 237 for IS) and positive ion electrospray ionization interface. Drying gas flow was set to 6 l/min and temperature to 350°. Nebuliser pressure and capillary voltage of the system were adjusted to 20 psi and 3500 V, respectively.
The volume of 10 µl of internal standard working solution was transferred into a 1.5 ml tube containing 100 μl rat blank plasma. Protein precipitation was performed by adding 200 μl of acetonitrile to each sample. The mixture was vortexed for 1.0 min and centrifuged for 10 min at 13,000 rpm. The supernatant (10 μl) was injected into the liquid chromatography‑mass spectrometry system for analysis. The standards were prepared in the same way.
The concentration‑time profile of each probe drug was analysed by Drug and Statistic (DAS) software (Version 2.0, Mathematical Pharmacology Professional Committee of China, China) and statistical analyses were tested by t‑test using Statistical Package for the Social Sciences (SPSS, Version 13.0, IBM Corporation, NY, USA). A value of P<0.05 was considered to be statistically significant.
After blood sample processing, the pharmacokinetics of four probe drugs were simultaneously determined by HPLC‑MS. Time‑concentration profiles of the four probes were shown in fig. 1 and pharmacokinetic parameters were shown in Table 1. From the figure and the table, the elimination half‑life t1/2z between control group and test group of four probe drugs were: tolbutamide 5.291±3.199 versus 7.698±3.910 h, phenacetin 5.052±0.836 versus 5.121±2.655 h, omeprazole 5.717±3.222 versus 4.883±1.553 h and bupropion 7.137±1.204 versus 6.524±0.928 h. The t1/2z of tolbutamide had significant difference between control group and test group, and that of phenacetin, omeprazole and bupropion did not have significant difference. On top of that, the increase in maximum concentration (Cmax) and Area under the curve (AUC(0-∞)) of tolbutamide, and the corresponding decrease in Clearance (CLz) suggested that Wuniu early tea could inhibit CYP2C9. However, the Cmax, AUC (0‑∞) and CLz of three other probe drugs in the test rats showed no statistically significant difference from the control rats. It demonstrated that Wuniu early tea does not have influence on CYP1A2, CYP2C19 and CYP2B6.
| Drug | Parameter | Control | Multiple dose |
|---|---|---|---|
| Tolbutamide | t1/2z(h) | 5.291 ± 3.199 | 7.698 ± 3.910* |
| Cmax(ng/ml) | 17678.051 ± 3460.945 | 30708.279 ± 6438.639** | |
| AUC (0‑∞) (ng/h/ml) | 246114.178 ± 28325.637 | 402478.216 ± 119104.064* | |
| Vz(l/kg) | 0.215 ± 0.101 | 0.115 ± 0.057* | |
| CLz (l/h/kg) | 0.021 ± 0.002 | 0.013 ± 0.004** | |
| Phenacetin | t1/2z(h) | 5.052 ± 0.836 | 5.121 ± 2.655 |
| Cmax(ng/ml) | 4770.104 ± 577.35 | 4779.309 ± 542.55 | |
| AUC (0‑∞) (ng/h/ml) | 4163.948 ± 621.719 | 3886.814 ± 738.559 | |
| Vz(l/kg) | 26.338 ± 3.287 | 29.95 ± 18.516 | |
| CLz (l/h/kg) | 3.653 ± 0.51 | 3.961 ± 0.805 | |
| Omeprazole | t1/2z(h) | 5.717 ± 3.222 | 4.883 ± 1.553 |
| Cmax(ng/ml) | 609.251 ± 88.857 | 620.629 ± 31.897 | |
| AUC (0‑∞) (ng/h/ml) | 2531.029 ± 2191.613 | 3083.02 ± 2654.874 | |
| Vz(l/kg) | 172.776 ± 61.639 | 175.912 ± 49.173 | |
| CLz (l/h/kg) | 16.245 ± 3.309 | 17.753 ± 2.576 | |
| Bupropion | t1/2z(h) | 7.137 ± 1.204 | 6.524 ± 0.928 |
| Cmax(ng/ml) | 327.402 ± 11.758 | 334.102 ± 11.241 | |
| AUC (0‑∞) (ng/h/ml) | 1729.908 ± 286.424 | 1624.336 ± 164.811 | |
| Vz(l/kg) | 89.273 ± 2.925 | 86.898 ± 7.527 | |
| CLz (l/h/kg) | 8.836 ± 1.504 | 9.302 ± 0.998 |
*P<0.05,**P<0.01 versus control
Table 1: Effects Of Wuniu Early Tea on Pharmacokinetic Parameters of the Four Probe Drugs (Mean±Sd, N‑6)
Probe drugs have been extensively used for phenotyping many different kinds of CYP450 activities, and this approach has been widely applied in field of drug metabolism and pharmacokinetics. A number of phenotyping cocktails have previously been proposed and evaluated in vivo and in vitro [11,18‑20]. The cocktail method that concurrently detects the activity of multiple CYP enzymes following simultaneous administrations of multiple CYP‑specific probe drugs offers several important advantages: (1) It enables real‑time assessment of the activity of various drug‑metabolising enzymes with a single experiment session; and (2) the confounding influence of intrasubject variability over time is minimised [21‑24]. Therefore, a new four‑drug cocktail including tolbutamide, phenacetin, omeprazole and bupropion to study the influence of natural products and new chemical drugs on the metabolising activity of CYP2C9, CYP1A2, CYP2C19 and CYP2B6, respectively, had been established in our laboratory. The cocktail demonstrated that the capacity of the four probe drugs for determining the activity of each enzyme is not affected by the potential for analytical interference from the co‑administered probe drugs or their metabolites. For the restrictions in our experimental conditions, the cocktail does not include two major CYP isoforms CYP3A4 and CYP2D6.
This study represents the first attempt to assess the potential effects of induction or inhibition on CYP450 activities including CYP2C9, CYP1A2, CYP2C19 and CYP2B6 in rats after repeated administration of Wuniu early tea. In addition, it also provides the helpful data to find out the metabolites of Wuniu early tea. The pharmacokinetic parameter estimates of each probe drug in vivo are reliable to evaluate the entirety of the drug–drug interaction in the drug metabolism than a vitro study. In our study, we found that the drug concentration and t1/2z of tolbutamide significantly increased (P<0.05) and the activity of CYP2C9 tended to be inhibited. The complicated mechanism of this effect of Wuniu early tea on the activity of CYP2C9 needs to be further studied. Another possibility is that the concentration of Wuniu early tea in present study might not be high enough to inhibit the activity of CYP2C9. The study in vitro is so critical that it helps us to understand the metabolic functioning of the new drug completely, and is widely carried out to make certain the effects of the new drug on CYP in medical researches [15,25,26]. So, the study of Wuniu early tea’s impact on CYP2C9 activity in vitro needs to be discussed in the future. Beyond that problem, the dose dependency and kinetics of Wuniu early tea on the activity of CYP2C9 in vivo and in vitro also remain to be studied.
CYP2C9 is one of the most abundant CYP enzymes in the human liver (~20% of hepatic total CYP content), where it biotransforms approximately 15% clinical drugs (>100 drugs), including a large number of drugs with narrow therapeutic ranges [27]. Tolbutamide have been well studied as marker compound for determining the metabolic activity of CYP2C9 in vitro as well as in vivo and is most commonly used in CYP2C9 phenotyping studies [21,28]. Wuniu early tea has been widely used to be the beverage for a long history. With the great use of Wuniu early tea as a thirst quencher, people should pay more attention to its side effects caused by drug– drug interactions when they are administrated with other drugs, especially with substrates of CYP2C9. Due to its inhibitive effect of CYP2C9, it is also of great important significance, to evaluate the clinical safety of Wuniu early tea.
Pharmacokinetic parameters may reflect hepatic metabolism of Wuniu early tea in vivo. The present study assessed the effects of induction and inhibition of Wuniu early tea on CYP2C9, CYP1A2, CYP2C19 and CYP2B6 by pharmacokinetic parameters of four selected probe drugs tolbutamide, phenacetin, omeprazole and bupropion, respectively. The results suggested that Wuniu early tea could inhibit CYP2C9, and did not influence on CYP1A2, CYP2C19 and CYP2B6. It will be useful for understanding the effects of induction or inhibition of Wuniu early tea on CYP2C9, CYP1A2, CYP2C19 and CYP2B6, as well as assessing the safety and drug interaction of coadministration with other drugs.
References
- Fugh‑Berman A, Ernst E. Herb‑drug interactions: Review and assessment of report reliability. Br J ClinPharmacol 2001;52:587‑95.
- Markowitz JS, Donovan JL, DeVane CL, Taylor RM, Ruan Y, Wang JS, et al. Effect of St John?s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500‑4.
- Huang SM, Hall SD, Watkins P, Love LA, Serabjit‑Singh C, Betz JM, et al. Drug interactions with herbal products and grapefruit juice:A conference report.ClinPharmacolTher 2004;75:1‑12.
- Streetman DS, Bertino JS Jr, Nafziger AN. Phenotyping of drug‑metabolizing enzymes in adults: A review of in vivo cytochrome P450 phenotyping probes. Pharmacogenetics 2000;10:187‑216.
- Peng SX, Cousineau M, Juzwin SJ, Ritchie DM. A 96‑well screen filter plate for high‑throughput biological sample preparation and LC‑MS/ MS analysis. Anal Chem 2006;78:343‑8.
- Ryu JY, Song IS, Sunwoo YE, Shon JH, Liu KH, Cha IJ, et al. Development of the Inje cocktail for high‑throughput evaluation of five human cytochrome P450 isoforms in vivo.ClinPharmacolTher 2007;82:531‑40.
- Li X, Chen X, Li Q, Wang L, Zhong D. Validated method for rapid inhibition screening of six cytochrome P450 enzymes by liquid chromatography‑tandem mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2007;852:128‑37.
- Zhang S, Song N, Li Q, Fan H, Liu C. Liquid chromatography/tandem mass spectrometry method for simultaneous evaluation of activities of five cytochrome P450s using a five‑drug cocktail and application to cytochrome P450 phenotyping studies in rats. J Chromatogr B AnalytTechnol Biomed Life Sci 2008;871:78‑89.
- Zhang W, Han F, Guo P, Zhao H, Lin ZJ, Huang MQ, et al. Simultaneous determination of tolbutamide, omeprazole, midazolam and dextromethorphan in human plasma by LC‑MS/MS ? A high throughput approach to evaluate drug‑drug interactions. J Chromatogr B AnalytTechnol Biomed Life Sci 2010;878:1169‑77.
- Liu Y, Jiao J, Zhang C, Lou J. A simplified method to determine five cytochrome P450 probe drugs by HPLC in a single run. Biol Pharm Bull 2009;32:717‑20.
- Otten JN, Hingorani GP, Hartley DP, Kragerud SD, Franklin RB. An in vitro, high throughput, seven CYP cocktail inhibition assay for theevaluation of new chemical entities using LC‑MS/MS. Drug MetabLett 2011;5:17‑24.
- Zhang LL, Zhang JR, Guo K, Ji H, Zhang Y, Jiang SX. Effects of fluoroquinolones on CYP4501A and 3A in male broilers. Res Vet Sci 2011;90:99‑105.
- Feidt DM, Klein K, Hofmann U, Riedmaier S, Knobeloch D, Thasler WE, et al. Profiling induction of cytochrome p450 enzyme activity by statins using a new liquid chromatography‑tandem massspectrometry cocktail assay in human hepatocytes. Drug MetabDispos 2010;38:1589‑97.
- Lu C, Gallegos R, Li P, Xia CQ, Pusalkar S, Uttamsingh V, et al. Investigation of drug‑drug interaction potential of bortezomibin vivo in female Sprague‑Dawley rats and in vitro in human liver microsomes. Drug MetabDispos 2006;34:702‑8.
- Chou YC, Chung YT, Liu TY, Wang SY, Chau GY, Chi CW, et al. The oxidative metabolism of dimemorfan by human cytochrome P450 enzymes. J Pharm Sci 2010;99:1063‑77.
- Tang H, Min G, Ge B, Li Y, Liu X, Jiang S. Evaluation of protective effects of Chi‑Zhi‑Huang decoction on Phase I drug metabolism of liver injured rats by cocktail probe drugs. J Ethnopharmacol 2008;117:420‑6.
- Nagata M, Hidaka M, Sekiya H, Kawano Y, Yamasaki K, Okumura M, et al. Effects of pomegranate juice on human cytochrome P450 2C9and tolbutamide pharmacokinetics in rats. Drug MetabDispos 2007;35:302‑5.
- Youdim KA, Lyons R, Payne L, Jones BC, Saunders K. An automated, high‑throughput, 384 well Cytochrome P450 cocktail IC50 assay using a rapid resolution LC‑MS/MS end‑point. J Pharm Biomed Anal 2008;48:92‑9.
- Turpault S, Brian W, Van Horn R, Santoni A, Poitiers F, Donazzolo Y, et al. Pharmacokinetic assessment of a five‑probe cocktail for CYPs1A2, 2C9, 2C19, 2D6 and 3A. Br J ClinPharmacol 2009;68:928‑35.
- Alden PG, Plumb RS, Jones MD, Rainville PD, Shave D.A rapid ultra ‑performance liquid chromatography/tandem mass spectrometric methodology for the in vitro analysis of pooled and cocktail cytochrome P450 assays. Rapid Commun Mass Spectrom 2010;24:147‑54.
- Tanaka E, Kurata N, Yasuhara H. How useful is the ?cocktail approach? for evaluating human hepatic drug metabolizing capacity using cytochrome P450 phenotyping probes in vivo? J Clin Pharm Ther 2003;28:157‑65.
- Stewart NA, Buch SC, Conrads TP, Branch RA. A UPLC‑MS/MS assay of the ?Pittsburgh cocktail?: Six CYP probe‑drug/metabolites from human plasma and urine using stable isotope dilution. Analyst 2011;136:605‑12.
- Frye RF, Matzke GR, Adedoyin A, Porter JA, Branch RA. Validation of the five‑drug ?Pittsburgh cocktail? approach for assessment of selective regulation of drug‑metabolizing enzymes. ClinPharmacolTher 1997;62:365‑76.
- Chainuvati S, Nafziger AN, Leeder JS, Gaedigk A, Kearns GL, Sellers E, et al. Combined phenotypic assessment of cytochrome p450 1A2, 2C9, 2C19, 2D6, and 3A, N‑acetyltransferase‑2, and xanthine oxidase activities with the ?Cooperstown 5+1 cocktail?. ClinPharmacolTher 2003;74:437‑47.
- Dostalek M, Jurica J, Pistovcakova J, Hanesova M, Tomandl J, Linhart I, et al. Effect of methamphetamine on cytochrome P450 activity. Xenobiotica 2007;37:1355‑66.
- Yao YM, Cao W, Cao YJ, Cheng ZN, Ou‑Yang DS, Liu ZQ, et al. Effect of sinomenine on human cytochrome P450 activity. ClinChimActa 2007;379:113‑8.
- Miners JO, Birkett DJ. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br J ClinPharmacol 1998;45:525‑38.
- Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA. Tolbutamide, flurbiprofen, and losartan as probes of CYP2C9 activity in humans. J ClinPharmacol 2003;43:84‑91.
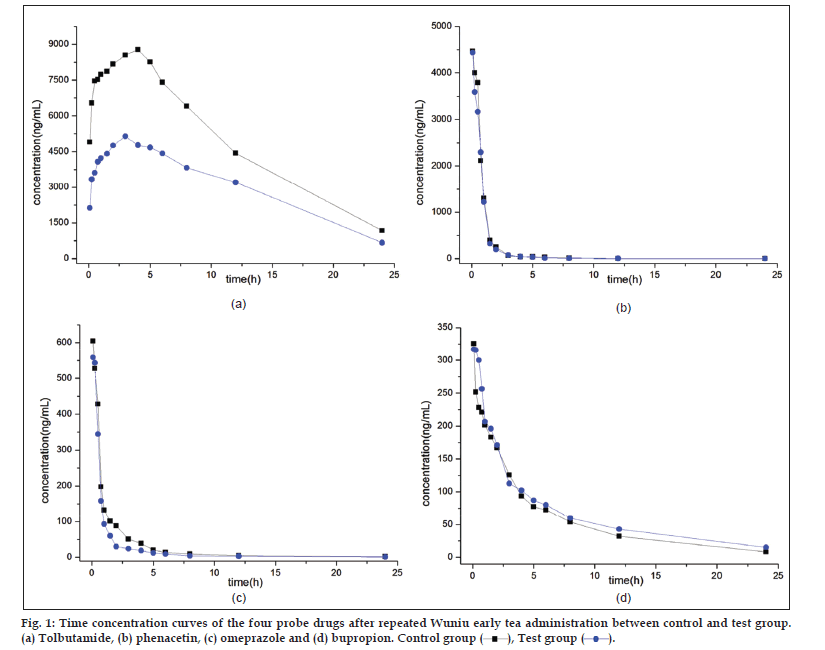
 ), Test group (
), Test group (  ).
).



