- *Corresponding Author:
- Mingyu Yang
Department of Chinese Medicine, Cangzhou Medical College, Cangzhou, Guangdong 061001, China
E-mail: 18833767766@163.com
| Date of Received | 14 April 2023 |
| Date of Revision | 20 December 2023 |
| Date of Acceptance | 03 May 2024 |
| Indian J Pharm Sci 2024;86(3):818-824 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study is to explore the effect of plantamajoside on malignant properties of MHCC97H cells and its possible mediating molecular mechanisms. Effects of high, median, and low concentrations of plantamajoside on MHCC97H cell malignant behaviors were assessed. Quantitative reverse transcriptase polymerase chain reaction analysis detected microRNA-3178 expression in tissue samples. Western blotting detected E-cadherin and N-cadherin protein levels. Plantamajoside led to a remarkable reduction in the proliferative, migrating and invasive capabilities of MHCC97H cells, which was accompanied with a diminution in N-cadherin protein levels as well as an augmentation in E-cadherin protein level and microRNA-3178 expression. Reduction of microRNA-3178 was noted in hepatocellular carcinoma samples vs. para-carcinoma tissues. Furthermore, microRNA-3178 overexpression demonstrated an ability to inhibit the effects on the invasion, metastasis, and proliferation of MHCC97H cells. Amusingly, microRNA-3178 silencing attenuated the plantamajoside-mediated inhibitory impact on malignant behaviours of MHCC97H cells. Plantamajoside treatment resulted in an upregulation of microRNA-3178, thus repressing MHCC97H cell invasion, metastasis, and proliferation.
Keywords
MHCC97H cells, plantamajoside, microRNA-3178, Plantago asiatica, hepatocellular carcinoma
Hepatocellular Carcinoma (HCC) is a complex malignant tumor with lethality, ranking 6th in global mortality rates[1]. Multiple factors contribute to the emergence of this disease, including fatty liver caused cirrhosis, carcinogenic toxins, viral infections, and miscellaneous genetic factors[2,3]. Interventions for HCC include surgical resection, transarterial chemoembolization, local ablation, sorafenib therapy, and liver transplantation[4]. Despite these robust therapeutic interventions, survival in patients with HCC remains sluggish[5,6]. Therefore, more effective drugs with minimal toxicity should be developed to improve overall survival in HCC patients.
Plantago asiatica L., a plant of the family Plantaginaceae, has the effects of clearing heat and drenching, expelling phlegm, diuretic and cooling blood[7]. Plantago contains a variety of active substances, among which Plantamajoside (PMS) is a representative component for evaluating the quality of Plantago in the 2020 edition of the Chinese Pharmacopoeia[8]. As confirmed by studies, PMS has anti-inflammatory[9], anti-oxidant[10], immunomodulatory[11], and anti-tumor properties[12]. For instance, improvement of Adenosine Monophosphate-activated Protein Kinase (AMPK)/Nuclear factor erythroid 2-related factor 2 (Nrf2) by PMS regulated hepatic lipid metabolism and immune dysregulation in Nonalcoholic Fatty Liver Disease (NAFLD) rats[11]. Inflammatory responses in Lipopolysaccharide (LPS) stimulated human gingival fibroblasts were attenuated by PMS through repression of the Phosphoinositide-3-Kinase/Protein Kinase B (PI3K/Akt) pathway[13]. PMS represses cancer cell metastasis and growth in manifold tumors, such as melanoma[14], cervical cancer[15], and HCC[16]. At present, the molecular mechanisms related to PMS-mediated inhibition of HCC have not been well elucidated.
MicroRNAs (miRNAs), a large family of single-stranded Ribonuclic Acid (RNA) molecules (18-25 nucleotides), play a critical role as post-transcriptional regulators[17]. Regarding the regulatory role of miRNAs in various gene expression levels, dysfunction or dysregulation of the expression of these molecules can lead to various diseases, including various types of cancer[18]. As observed across miscellaneous studies, miRNA dysregulation has been recognized as a causative mechanism behind HCC tumorigenesis[19]. PMS has been reported to decrease S100 calcium-binding protein A16 (S100A16) expression by elevating miR-711 expression, inhibiting bladder cancer cell metastasis and proliferation[20] and whether PMS affects the malignant behavior of HCC cells by regulating miRNA levels has not been well elucidated. However, a tumor-suppressive miRNA, miR-3178 has been reported in other cancers. Dysregulation of miR-3178 has been associated with the reversal of gemcitabine resistance in pancreatic cancer[21]. miR-3178 also refers to the carcinogenesis of HCC[22], breast cancer[23] and gastric cancer[24]. Currently, it is unclear whether PMS affects HCC cell malignancy by regulating the levels of miR-3178.
Thus in this study, whether PMS inhibits the malignant behaviour of HCC cells by mediating miR-3178 expression was addressed.
Materials and Methods
Cell culture, experimental grouping and transfection:
MHCC97H cells (Honsun Biological Technology Co., Ltd.) were maintained in Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich, USA) supplemented with 10 % Fetal Bovine Serum (FBS).
MHCC97H cells were incubated in DMEM medium containing High (H) (200 μg/ml), Low (L) (50 μg/ ml), and Moderate (M) (100 μg/ml) concentrations of PMS (Shanghai Yuanye Biological Technology Co., Ltd.) and was categorized as PMS-L, PMS-M and PMS-H groups, respectively.
For transient transfection of miR-Negative Control (NC), miR-3178 mimics, anti-miR-NC, anti-miR-3178 (Ribobio, Guangzhou, China) and Lipofectamine™ 3000 (Invitrogen) was utilized. After successful transfection, the cells were cultured in DMEM medium containing PMS (200 μg/ml) for 24 h and labelled as PMS+anti-miR-NC and PMS+anti-miR-3178 groups, respectively.
Cell proliferation analysis:
After incubation of MHCC97H cells (3×103 cells/ well) for 24 h, 10 μl of the Cell Counting Kit-8 (CCK-8) solution (Solarbio, Beijing, China) was added. By applying a plate reader (Tecan, Group Ltd., Switzerland), we tested the Optical Density (OD) at 450 nm in each well. Calculation of cell proliferation inhibition rate was performed by calculating, (OD control-OD Experiment)/(OD control-OD blank)×100 %.
For clone formation assay, MHCC97H cells (500 cells/well) were inoculated and continued to be cultured until visible cell clones. Fixation of cells was performed by adding 500 μl of methanol (Solarbio Life Science, China). After 20 min, 1 % crystal violet staining solution staining (Solarbio) was added. Counting of the number of cell clones (≥50 cells as 1 clone) was carried out under the microscope.
Wound healing assay:
Inoculation of approximately 1×105 MHCC97H cells/well into 6-well plates was done. When the cells were grown fully, wounding was formed by scribing a line across the cell monolayer with a sterile pipette tip (200 μl). Imaging was performed under a microscope at 0 and 24 h. ImageJ software was applied to detect the migration distance. Calculation of the healing rate was done using the formula, (Width 0 h-width 24 h)/(Width 0 h×100 %).
Transwell assay for cell invasion:
Diluted Matrigel (300 μg/ml and 45 μl/well) was added to the upper chamber (Solarbio). MHCC97H cells (1×105 cells/well) were added to the upper chamber and medium containing 10 % FBS (500 μl) was added to the lower chamber subsequently. Following 24 h of incubation, fixation was carried out with paraformaldehyde (Solarbio). After 10 min of staining with 0.1 % crystal violet the cells were observed under the microscope.
Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR):
Isolation of total RNA was accomplished using Total RNA Isolation (TRIzol) reagent (Thermo, USA). Using the miScript® II RT kit (Applied Biosystems, USA), complementary Deoxyribonucleic Acid (cDNA) was synthesized following the manufacturer’s recommendations. miR-3178 expression was analyzed using qRT-PCR using the TaqMan miRNA assay (Applied Biosystems). Small nuclear RNA (snRNA) U6 was used for normalization and 2-ΔΔCt method was applied for the determination of the relative expression levels.
Western blotting:
Lysis of MHCC97H cells was performed with Radio-Immunoprecipitation Assay (RIPA) buffer (Beyotime, Shanghai, China). Following the detection of protein concentration, 40 μg of protein samples were subjected to Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE). The membranes were incubated with the primary antibodies against Epithelial (E)-cadherin (1:1000, Abcam, Massachusetts, USA), Neural (N)-cadherin (1:1000, Abcam), and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:3000, Abcam). Protein expression was detected by chemiluminescence using SuperSignalTM West Pico PLUS (Thermo Fisher Scientific, Inc.). Analysis of the grey values of each band was performed applying ImageJ software.
Statistical analysis:
The Graph Pad Prism 8 program (Graph Pad, Inc., San Diego, CA, USA) was used for the statistical analysis. The data was submitted to normalization analysis (Shapiro-Wilk test). Statistical significance was determined by student’s t test. Comparisons were made in multiple comparisons using one-way Analysis of Variance (ANOVA) procedures. Data were considered significant at p≤0.05.
Results and Discussion
In contrast to the control group, PMS resulted in increased proliferation inhibition as well as reduced number of clone formation in MHCC97H cells in a dose-dependent manner (Table 1).
| Group | Inhibitory rate (%) | Number of cell clone formation (n) |
|---|---|---|
| Control | 0.00±0.00 | 96.82±6.25 |
| PMS-L | 25.54±2.11* | 79.07±5.61* |
| PMS-M | 45.16±4.21*# | 61.25±5.01*# |
| PMS-H | 64.17±5.59*#& | 44.15±3.92*#& |
| F | 508.089 | 167.128 |
| p | 0.000 | 0.000 |
Note: &p<0.05, #p<0.05 and *p<0.05 with respect to PMS-M, PMS-L and control group respectively
Table 1: PMS Repressed Mhcc97H cell proliferation (N=9)
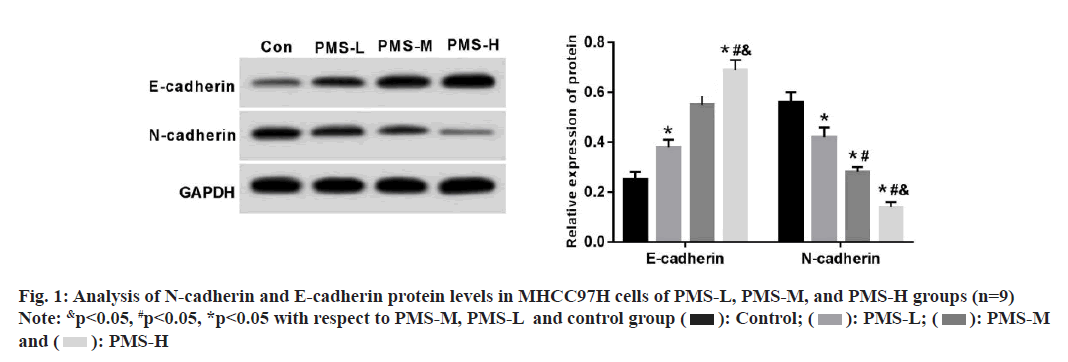
Further experiments yielded that PMS caused a dose-dependent decrease in wound healing rate and invasive capacity of MHCC97H cells compared to the control group (Table 2). Simultaneously, PMS caused lowering of N-cadherin protein levels as well as an increase of E-cadherin protein levels, and these changes occurred in a dose-dependent pattern (fig. 1).
| Group | Wound healing rate (%) | Number of cell invaded (n) |
|---|---|---|
| Control | 72.14±5.64 | 125.47±11.24 |
| PMS-L | 55.11±4.65* | 91.39±8.03* |
| PMS-M | 39.35±3.71*# | 72.57±6.08*# |
| PMS-H | 23.16±2.26*#& | 54.27±5.17*#& |
| F | 219.720 | 130.794 |
| p | 0.000 | 0.000 |
Note: &p<0.05, #p<0.05 and *p<0.05 with respect to PMS-M, PMS-L and control group respectively
Table 2: PMS restrained Mhcc97H cell metastasis and invasion (N=9)
In comparison to para-cancerous tissues, miR-3178 was reduced markedly in HCC samples (Table 3). Comparing with the control group, PMS provoked a remarkable elevation of miR-3178 expression in MHCC97H cells in a dose-dependent pattern (Table 4).
| Group | miR-3178 |
|---|---|
| Para-cancerous tissues | 1.00±0.12 |
| HCC tissues | 0.37±0.04* |
| t | 31.891 |
| p | 0.000 |
Note: *p<0.05, in comparison with para-cancerous tissues
Table 3: Levels of miR-3178 in HCC samples (N=41)
| Group | miR-3178 |
|---|---|
| Control | 1.00±0.00 |
| PMS-L | 1.72±0.13* |
| PMS-M | 2.55±0.22*# |
| PMS-H | 3.11±0.26*#& |
| F | 232.677 |
| p | 0.000 |
Table 4: PMS Elevated miR-3178 Levels in Mhcc97H Cells (N=9)
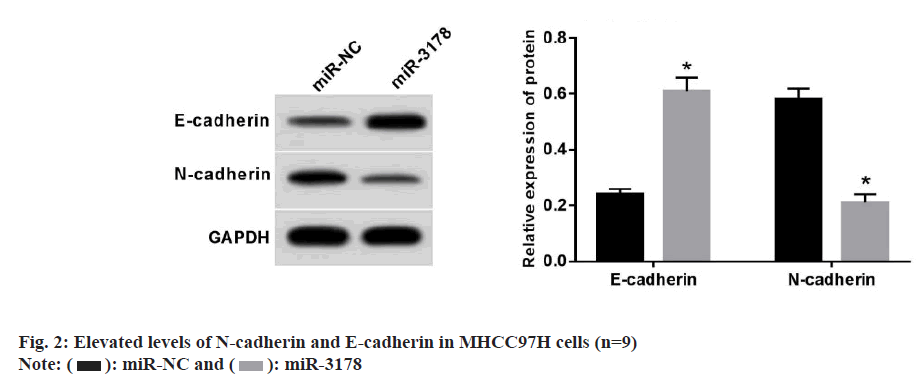
The introduction of miR-3178 expression resulted in a prominent degradation of the proliferative, migratory, and invasive capacities of MHCC97H cells when compared to the miR-NC group (Table 5). A strongly elevated levels of E-cadherin protein and reduced levels of N-cadherin protein levels were observed in miR-3178 mimic-transfected MHCC97H cells (fig. 2).
| Group | miR-3178 | Inhibitory rate (%) | Number of cell clone formation (n) | Wound healing rate (%) | Number of invaded cells (n) |
|---|---|---|---|---|---|
| miR-NC | 1.00±0.00 | 5.88±0.48 | 99.34±7.48 | 73.15±6.35 | 127.51±12.29 |
| miR-3178 | 3.24±0.27* | 50.16±4.75* | 52.01±4.91* | 32.61±3.12* | 60.68±5.45* |
| t | 24.889 | 27.825 | 15.869 | 17.190 | 14.913 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, with respect to miR-NC
Table 5: miR-3178 overexpression repressed Mhcc97H cell invasion, metastasis and proliferation (N=9)
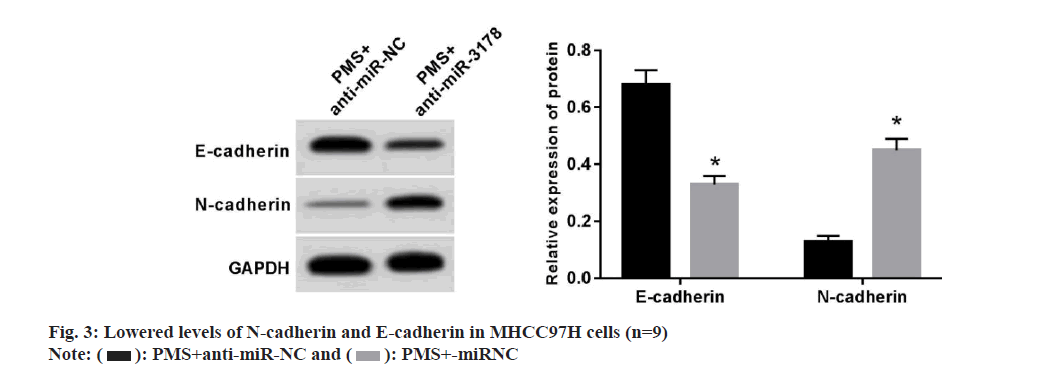
MHCC97H cells in the PMS+anti-miR-NC group showed increased proliferative, migratory, and invasive potentials by comparison with those in the PMS+anti-miR-NC group (Table 6) along with diminished levels of E-cadherin protein and augmented levels of N-cadherin protein (fig. 3).
| Group | miR-3178 | Inhibitory rate (%) | Number of cell clone formation (n) | Wound healing rate (%) | Number of cell invaded (n) |
|---|---|---|---|---|---|
| PMS+anti-miR-NC | 1.00±0.00 | 66.83±4.84 | 43.28±3.64 | 21.97±2.21 | 51.78±4.72 |
| PMS+anti-miR-3178 | 0.46±0.04* | 27.15±2.63* | 82.05±5.62* | 61.26±5.03* | 106.64±11.52* |
| t | 40.500 | 21.611 | 17.371 | 21.454 | 13.220 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, with respect to PMS+anti-miR-NC
Table 6: Repression of miR-3178 expression retrograded with PMS-Mediated Impacts on Mhcc97H Cell Invasion, Metastasis and Proliferation (N=9)
Early symptoms of HCC are relatively insidious, leading to poor prognosis, and there are no effective drugs and treatments to control the metastasis of HCC cells. Prior investigations have shown that some traditional Chinese medicines can facilitate HCC cell apoptosis and prohibit the metastasis and growth of HCC cells[25,26]; aberrantly expressed miRNAs were identified in HCC. Considerable studies have been conducted to confirm that miRNAs can modulate the biological behaviours of HCC cells and may also work as potential targets for HCC treatment[27,28]. However, whether miRNAs can be used as potential targets for drug treatment of HCC needs to be elucidated.
A previous study revealed that PMS inhibited HCC cell proliferation and metastasis[16]. As a result of inhibiting Akt/Glycogen Synthase Kinase-3 Beta (GSK3β) signaling, PMS fostered metformin-induced autophagy and apoptosis in HCC cells[29]. Similarly, PMS was shown to restrain the growth and metastasis of malignant melanoma[14]. Lowering of Matrix Metalloproteinase (MMP)-9/2 activities by PMS inhibited lung metastasis and tumor growth in breast cancer[30]. Inhibition of Akt and p38 Mitogen-Activated Protein Kinases (MAPK) phosphorylation by PMS regulated apoptosis and proliferation in lung cancer[31]. Here, PMS raised the rate of proliferative inhibition of MHCC97H cells and reduced the clone-forming capability in a concentration-dependent pattern, suggesting that PMS could regulate the proliferative capability of MHCC97H cells. At Epithelial-Mesenchymal Transition (EMT) process, N-cadherin and E-cadherin belong to a marker of mesenchymal cells and epithelial cells, respectively. Dysregulation of N-cadherin and E-cadherin can drive the EMT transition, thereby encouraging cell metastasis[32]. Findings of the present study were indicative of the fact that PMS treatment brought about a decrease in MHCC97H cell invasion and metastasis in a concentration-dependent pattern, accompanied by lowering of N-cadherin protein levels and up-regulation of E-cadherin protein levels, suggesting that PMS suppressed the invading and migrating abilities of HCC cells.
Downregulation of miR-3178 contributed to gastric cancer cell invasion[24]. By targeting Tumor necrosis factor Receptor Associated Factor 3 (TRAF3), miR-3178 suppressed the gastric cancer advancement[33], with low miR-3178 levels in breast cancer[34,35]. According to the results of current investigation, miR-3178 levels in HCC tissues were lower. With increase in PMS concentrations, miR-3178 expression in MHCC97H cells also increased progressively, suggesting that PMS might have an anti-cancer action by upregulating miR-3178 expression in HCC. Meanwhile, we found that miR-3178 overexpression restricted MHCC97H cell invasion, metastasis, and proliferation, whereas suppression of miR-3178 attenuated the inhibiting effects mediated by PMS, suggesting that PMS may regulate HCC cell growth and metastasis by increasing miR-3178 expression.
Collectively, PMS might restrict malignant behaviours of MHCC97H cells by upregulating miR-3178. This study provides a new direction regarding the therapeutic drug development for HCC and lays down the experimental foundation for further revealing about the mechanism of action of PMS against HCC. However, the underlying molecular mechanisms of PMS-mediated inhibitory effects on HCC still need to be further investigated.
Conflict of interests:
The authors declared no conflict of interests.
References
- Li Z, Zhang Y, Guo R, Liu ZL, Wang H. Bibliometric study of immunotherapy for hepatocellular carcinoma. Front Immunol 2023;14:1-22.
- Patmore LA, Katwaroe WK, van der Spek D, Choi HS, Patel K, Brakenhoff S, et al. Association between the presence of metabolic comorbidities and liver-related events in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2023;21(12):3089-96.
- Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol 2020;72(2):215-29.
- Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, et al. Management of hepatocellular carcinoma: A review. JAMA Surg 2023;158(4):410-20.
- Rajendran L, Ivanics T, Claasen MP, Muaddi H, Sapisochin G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin Mol Hepatol 2022;28(1):1-6.
- Wang J, Wu R, Sun JY, Lei F, Tan H, Lu X. An overview: Management of patients with advanced hepatocellular carcinoma. Biosci Trends 2022;16(6):405-25.
- Wen SY, Wei BY, Ma JQ, Wang L, Chen YY. Phytochemicals, biological activities, molecular mechanisms, and future prospects of Plantago asiatica L. J Agric Food Chem 2022;71(1):143-73.
- Chinese Pharmacopoeia Commission: Pharmacopoeia of the People's Republic of China; 2020.
- Lin S, Lu J, Chen Q, Jiang H, Lou C, Lin C, et al. Plantamajoside suppresses the activation of NF-κB and MAPK and ameliorates the development of osteoarthritis. Int Immunopharmacol 2023;115:109582.
- Li Y, Gan L, Li GQ, Deng L, Zhang X, Deng Y. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography-mass spectrometry. J Pharm Biomed Anal 2014;89:251-6.
[Crossref] [Google Scholar] [PubMed]
- Wu JM, Zhaori G, Mei L, Ren XM, Laga AT, Deligen B. Plantamajoside modulates immune dysregulation and hepatic lipid metabolism in rats with nonalcoholic fatty liver disease via AMPK/Nrf2 elevation. Kaohsiung J Med Sci 2023;39(8):801-10.
- Li X, Chen D, Li M, Gao X, Shi G, Zhao H. Plantamajoside inhibits lipopolysaccharide-induced epithelial-mesenchymal transition through suppressing the NF-κB/IL-6 signaling in esophageal squamous cell carcinoma cells. Biomed Pharmacother 2018;102:1045-51.
[Crossref] [Google Scholar] [PubMed]
- Liu F, Huang X, He JJ, Song C, Peng L, Chen T, et al. Plantamajoside attenuates inflammatory response in LPS-stimulated human gingival fibroblasts by inhibiting PI3K/Akt signaling pathway. Microb Pathog 2019;127:208-11.
- Wang Y, Liu M, Chen S, Wu Q. Plantamajoside represses the growth and metastasis of malignant melanoma. Exp Ther Med 2020;19(3):2296-302.
- Zuo X, Li L, Sun L. Plantamajoside inhibits hypoxia-induced migration and invasion of human cervical cancer cells through the NF-κB and PI3K/Akt pathways. J Recept Signal Transduct 2021;41(4):339-48.
- Yin W, Xu J, Li C, Dai X, Wu T, Wen J. Plantamajoside inhibits the proliferation and epithelial‐to‐mesenchymal transition in hepatocellular carcinoma cells via modulating hypoxia‐inducible factor‐1α‐dependent gene expression. Cell Biol Int 2020;44(8):1616-27.
- Hwang H, Chang HR, Baek D. Determinants of functional microRNA targeting. Mol Cells 2023;46(1):21.
- Alsaab HO, Abdullaev B, Alkhafaji AT, Alawadi AH, Jahlan I, Bahir H, et al. A comprehension of signaling pathways and drug resistance; an insight into the correlation between microRNAs and cancer. Pathol Res Pract 2023;251:154848.
- Hajizadeh M, Hajizadeh F, Ghaffarei S, Doustvandi MA, Hajizadeh K, Yaghoubi SM, et al. MicroRNAs and their vital role in Apoptosis in Hepatocellular carcinoma: MiRNA-based diagnostic and treatment methods. Gene 2023;888:147803.
- Geng H, Dingwen G, Jinlun F, Ling L. Plantamajoside inhibits the proliferation and migration of bladder cancer cells by regulating the expression of miR-711/S100A16 signaling pathway. J Shanxi Med Univ 2022;53(12):1526-30.
- Gu J, Huang W, Wang X, Zhang J, Tao T, Zheng Y, et al. Hsa-miR-3178/RhoB/PI3K/Akt, a novel signaling pathway regulates ABC transporters to reverse gemcitabine resistance in pancreatic cancer. Mol Cancer 2022;21(1):1-17.
- Li W, Shen S, Wu S, Chen Z, Hu C, Yan R. Regulation of tumorigenesis and metastasis of hepatocellular carcinoma tumor endothelial cells by microRNA-3178 and underlying mechanism. Biochem Biophys Res Commun 2015;464(3):881-7.
- Kong P, Chen L, Yu M, Tao J, Liu J, Wang Y, et al. miR-3178 inhibits cell proliferation and metastasis by targeting Notch1 in triple-negative breast cancer. Cell Death Dis 2018;9(11):1-12.
- Wu JC, Liu ZH, Ding X, Ke RS. MiR-3178 as a prognostic indicator and tumor suppressor of gastric cancer. Irish J Med Sci 2022;191(1):139-45.
- Yin S, Jin W, Qiu Y, Fu L, Wang T, Yu H. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J Hematol Oncol 2022;15(1):1-6.
- Liu JS, Huo CY, Cao HH, Fan CL, Hu JY, Deng LJ, et al. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine 2019;61:1-12.
- Qu L, Tian Y, Hong D, Wang F, Li Z. Mig-6 inhibits autophagy in HCC cell lines by modulating miR-193a-3p. Int J Med Sci 2022;19(2):338-51.
- Komoll RM, Hu Q, Olarewaju O, von Döhlen L, Yuan Q, Xie Y, et al. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol 2021;74(1):122-34.
- Wang Z, Zuo J, Zhang L, Zhang Z, Wei Y. Plantamajoside promotes metformin-induced apoptosis, autophagy and proliferation arrest of liver cancer cells via suppressing Akt/GSK3β signaling. Hum Exp Toxicol 2022;41:1-13.
- Pei S, Yang X, Wang H, Zhang H, Zhou B, Zhang D, et al. Plantamajoside, a potential anti-tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase-9 and 2. BMC Cancer 2015;15(1):1-2.
- Li Y, Han R, Cao W. Plantamajoside modulates the proliferation, stemness, and apoptosis of lung carcinoma via restraining p38MAPK and Akt phosphorylation. Transl Cancer Res 2020;9(6):1-14.
- Loh CY, Chai JY, Tang TF, Wong WF, Sethi G, Shanmugam MK, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019;8(10):1-33.
- Zou M, Wang F, Jiang A, Xia A, Kong S, Gong C, et al. MicroRNA‐3178 ameliorates inflammation and gastric carcinogenesis promoted by Helicobacter pylori new toxin, Tip‐α, by targeting TRAF3. Helicobacter 2017;22(2):1-14.
- Zhou Q, Zeng H, Ye P, Shi Y, Guo J, Long X. Differential microRNA profiles between fulvestrant-resistant and tamoxifen-resistant human breast cancer cells. Anticancer Drugs 2018;29(6):539-48.
- Tan HP, Guo Q, Hua G, Chen JX, Liang JC. Inhibition of endoplasmic reticulum stress alleviates secondary injury after traumatic brain injury. Neural Regen Res 2018;13(5):827-36.
 ): Control; (
): Control; ( ): PMS-L; (
): PMS-L; ( ): PMS-M
and (
): PMS-M
and ( ): PMS-H
): PMS-H