- *Corresponding Author:
- Houxiang Qin
Department of Pharmacy,
Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province 310009,
China
E-mail: 0919673@zju.edu.cn
| Date of Received | December 2022 |
| Date of Revision | 15 October 2023 |
| Date of Acceptance | 27 March 2024 |
| Indian J Pharm Sci 2024;86(2):502-508 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of liraglutide on myocardial injury in acute myocardial infarction rats by regulating phosphoinositide 3 kinase/protein kinase B signaling. Sprague-Dawley rats were randomly divided into sham group, model group, liraglutide group (0.6 mg/kg liraglutide), LY294002 (phosphoinositide 3 kinase inhibitor) (1.5 mg/kg LY294002) and liraglutide+LY294002 (LY294002 after liraglutide), with 12 rats in each group. Except for the sham group, the acute myocardial infarction rat model was constructed by coronary artery ligation. After successful mold making, the drug group was treated according to the administration method of each group, and the group was injected with equal amount of normal saline intraperitoneally for 2 w. Cardiac function was measured by echocardiography, hemodynamics were recorded by polygraph, myocardial histopathological changes were detected by hematoxylin and eosin staining, the degree of fibrosis was observed by Masson staining, and the expression of autophagy-related factors and proteins related to phosphoinositide 3 kinase/protein kinase B signaling pathway was detected in rats by Western blot. As compared to the sham group, a significant decrease in cardiac function, the degree of myocardial tissue damage and the degree of fibrosis, the expression of autophagy-related proteins and phosphoinositide 3 kinase/protein kinase B pathway-related proteins was significantly decreased; compared to the model group, the cardiac function, degree of fibrosis in liraglutide rats, the expression of autophagy-related proteins and phosphoinositide 3 kinase/protein kinase B pathway-related proteins was significantly increased; in contrast to the liraglutide group, cardiac function was significantly reduced in the liraglutide+LY2940002 group, the degree of myocardial tissue damage and the degree of fibrosis, the expression of autophagy-related proteins and phosphoinositide 3 kinase/protein kinase B pathway-related proteins was significantly decreased; compared with the LY2940002 group, the extent of cardiac function, myocardial tissue damage and fibrosis were alleviated in liraglutide+LY2940002 group rats, the expression of autophagy-related proteins and phosphoinositide 3 kinase/protein kinase B pathway-related proteins was significantly increased. Liraglutide can improve the myocardial injury and protect the cardiac function by activating the phosphoinositide 3 kinase/protein kinase B signal pathway in acute myocardial infarction rats.
Keywords
Liraglutide, acute myocardial infarction, phosphoinositide 3 kinase, protein kinase B
Acute Myocardial Infarction (AMI) is a coronary artery acute, persistent hypoxia caused by myocardial damage, and acute mood, physiological stress, overwork, overeating and other factors, often accompanied by heart dysfunction, arrhythmia, heart failure, shock, sternal pain, adverse symptoms such as mental disorder, seriously affect the quality of life and life health[1,2]. Glucagon Like Peptide-1 (GLP- 1), a member of the proglucagon-derived peptide family, is an insulin hormone that regulates blood glucose[3]. Liraglutide is a GLP-1 analogue that has been reported to reduce the cardiac rupture and infarct range in both normal and diabetic mice[4], and had a significant improvement in rats with AMI[5]. Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/AKT) pathway is involved in various physiological processes such as cell proliferation, metabolism, secretion, neuroprotection, and cardio protection[6,7]. Previous studies have found that the activation of the PI3K/Akt signaling pathway can promote the repair and healing of myocardial tissue and significantly improve myocardial infarction[8]. Liraglutide can activate the PI3K/AKT/Glycogen Synthase Kinase-3 Beta (GSK 3β) pathway to exert neuroprotective effects after hypoxic-ischemic brain injury in neonatal rats[9]. However, the effect of liraglutide on PI3K/AKT pathway in AMI disease is rarely reported, so this paper explores the effect of liraglutide on regulating PI3K/AKT pathway on myocardial injury in AMI rats based on previous studies, and provides new ideas for clinical treatment of AMI.
Materials and Methods
Animals:
Specific Pathogen Free (SPF) grade Sprague- Dawley (SD) male rats were purchased from Huazhong Agricultural University, with the production license number SYXK (E) 2020- 0084, and their body weight was about 210 g. Experimental rats were raised in the animal room of our hospital (SYXK (E) 2018-0066), free food and drinking, 12 h, the animal room temperature at about 25°, the relative humidity at about 50 %, the animal room was cleaned every day, once a week, the animal ethics committee approved (batch number: 20200927), in the experiment according to the "3R" principle of humanitarian care.
Main reagents and instruments:
Liraglutide (98 % purity, goods number: IL1630), LY294002 (PI3K inhibitor), Masson staining kit, high efficiency Radioimmunoprecipitation Assay (RIPA) lysate (B-0294, G1346, R0010) were all purchased from Beijing Solaibao Technology Co., Ltd.; Light Chain 3 II (LC3II), Beclin-1, PI3K, Akt, β-actin antibodies, and sheep anti-rabbit Immunoglobulin G (IgG) antibodies (ab192890, ab210498, ab278545, ab8805, ab8227, and ab150077) were purchased from Abcam; Creatine Kinase Isoenzyme (CK-MB), myocardial injury marker (cardiac Troponin I (cTnI)) Enzyme-Linked Immunosorbent Assay (ELISA) kit (commodity numbers: ml059533 and ml059111) were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd.; VentElite Small animal ventilator was purchased from Harvard Instruments; DMD108 Optical microscope was purchased in Leica, Germany; P3Plus Polyguide physiological recorder was purchased from ponemah; SpectraMax iD3 Multi-function microplate reader was purchased from molecular devices.
Methods:
Preparation and group administration of animal models: Build AMI rat model, all rats preoperative fasting water for 12 h[10], after anesthesia fixed in the operating table, connected to small animal ventilator, lead electrocardiogram, cut the left margin of rat sternum 3rd and 4th intercostal skin, open the chest to expose the heart to left coronary anterior descending artery ligation with sterile suture, distal myocardial tissue rapid ischemia white, electrocardiogram ST elevation for ligation, chest air suture skin, observed that the rat breathing smoothly, normal Heart Rate (HR) after feeding in the cage to continue for 2 w. Another 12 rats were selected for sham group, and only the hearts were exposed without ligation. If the rats die or do not meet the standards, the spare rats should be selected for mold treatment. The number of rats is not less than 48, and the rats surviving for 2 w will be successful.
The 48 rats were divided into model group and liraglutide group (0.6 mg/kg liraglutide)[11], LY294002 (PI3K inhibitor) (1.5 mg/kg LY294002) [12], liraglutide+LY294002 group (LY294002 after liraglutide injection), sham group and model group injected equivalent (4 ml) of saline for 2 w.
Measurement of cardiac function indicators, hemodynamic indicators and tissue specimen collection in rats: At 24 h after the last dose, each groups of rats were fixed and echocardiography. The Left Ventricular Ejection Fraction (LVEF), short axis shortening rate of Left Ventricular Fractional Shortening (LVFS), and Left Ventricular Systolic Blood Pressure (LVSP) were recorded. After the echocardiography examination, aortic blood was collected about 4 ml, frozen for 30 min, centrifuged at 3000 r/min for 15 min, and serum was collected to determine the levels of CK-MB and cTnI in rat serum according to the operation steps of CK-MB and cTnI ELISA kit.
The right common carotid artery was isolated and the distal heart was ligated and packed with heparin (0.3 %) and the catheter was inserted into the ascending aorta and carotid artery. After the pressure value was observed for 10 min, the maximum rate of decrease in the left indoor pressure was recorded by polyguide physiological recorder (-dp/dtmax), maximum rise rate of the left indoor pressure (+dp/dtmax), HR, Left Ventricular End Diastolic Pressure (LVEDP), and Left Ventricular End Systolic Pressure (LVESP).
After rats were sacrificed, some for Hematoxylin and Eosin (H&E) staining observation, some for Masson staining, and some for the determination of autophagy-related proteins and PI3K/AKT pathway related proteins.
H&E staining and Masson staining in rat myocardial tissue: Take appropriate amount of rat myocardial tissue, fixed with 4 % paraformaldehyde for 24 h, after alcohol dehydration, xylene transparent paraffin embedded, to wax block after cooling solidification section, the paraffin section with xylene, different concentration gradient alcohol solution, distilled water, then by dyeing, water, eosin, alcohol and xylene after drying, sealing in the optical microscope analysis and take pictures, specific steps refer to the H&E dyeing kit instructions.
Take appropriate amount of rat myocardial tissue, fixed with 10 % of formalin, conventional paraffin embedded and slice processing, refer to the Masson staining kit instructions for dyeing processing, mainly medium dye, blue dye, hematein dye, differentiation dye, phosphomolybdic acid treatment, aniline blue dye liquid, after dehydration, transparent, neutral gum sealing steps, observed in the optical microscope analysis and photo.
Detection of autophagy-related factors and PI3K/AKT pathway protein expression in rat myocardial tissue: Appropriate amount of rat myocardial tissue was obtained, shear it into pieces at low temperatures, RIPA lysate was added to extract the total proteins, protein concentration in myocardial tissue was determined using a Bicinchoninic Acid (BCA) protein concentration determination kit, add protein solution of each group and denaturation, after cooling, 20 μl of each protein was isolated for electrophoretic experiments, the isolated proteins were transferred to a polyvinylidene difluoride membrane by the wet spin method, cut off the target protein and placed it in the incubation box, Add rabbit source LC3II (1:2000), Beclin-1 (1:1000), PI3K (1:300), AKT (1:250), β-actin (1:1000) primary antibodies, incubated overnight at 4°, horseradish peroxidelabeled sheep anti-rabbit IgG secondary antibody (1:2000) was subsequently added, was incubated at room temperature for 1 h. After color development, fix for photo analysis.
Statistical methods:
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) 22.0 software, and experimental data met normal distribution, all were expressed as mean±standard deviation (x̄ ±s), comparisons between groups by one-way Analysis of Variance (ANOVA), and further comparisons between two groups using Least Significant Difference (LSD)-t test with p<0.05 as statistically significant.
Results and Discussion
Compared to the sham surgery group, LVEF, LVSP, and LVFS values and the serum levels of CK-MB and cTnI in the model group, were significantly reduced (p<0.05); compared to the model group, the serum levels of CK-MB, cTnI in the liraglutide group, LVEF, LVSP, and LVFS values increased significantly (p<0.05). In contrast to the liraglutide group, significant increase in serum levels of CK-MB and cTnI in the liraglutide+LY2940002 group, the LVEF, LVSP, and LVFS values were significantly reduced (p<0.05); compared with the LY2940002 group, the serum levels of CKMb, cTnI in the liraglutide+LY2940002 group, the LVEF, LVSP, and LVFS values increased significantly (p<0.05) as shown in Table 1.
| Group | CK-Mb (U/l) | cTnI (mg/l) | LVEF (%) | LVSP (mmHg) | LVFS (%) |
|---|---|---|---|---|---|
| Sham surgery | 21.94±4.57 | 0.31±0.06 | 57.25±8.92 | 135.49±20.22 | 28.66±3.97 |
| Model set | 55.19±14.99a | 2.08±0.49a | 31.11±5.74a | 77.13±13.10a | 12.59±2.62a |
| Liraglutide | 27.37±5.43b | 0.55±0.18b | 48.51±7.04b | 109.39±16.44b | 24.46±3.77b |
| LY2940002 | 75.70±15.11c | 2.73±0.64c | 25.16±3.90c | 64.31±12.09c | 18.09±2.90c |
| Liraglutide+LY2940002 | 38.80±13.49cd | 1.17±0.31cd | 40.47±6.37cd | 92.15±15.61cd | 20.93±2.44cd |
Note: When compared to the sham surgery group, ap<0.05; compared to the model group, bp<0.05; compared to the liraglutide group, cp<0.05 and compared with the LY2940002 group, dp<0.05
Table 1: Comparison of Cardiac function indexes of rats in each group (X̄±S, N=12)
Compared with the sham group, rats in the model group had significantly higher LVEDP values, and had LVESP, +dp/dtmax, -dp/ dtmax, HR was significantly lower (p<0.05); compared with the model group, LVESP, +dp/ dtmax, -dp/dtmax, HR was significantly increased (p<0.05); compared with liraglutide group, LVEDP+LY2940002 group, LVESP, +dp/dtmax, -dp/ dtmax, HR values were significantly lower (p<0.05); liraglutide+LY2940002 rats, LVESP, +dp/dtmax, -dp/dtmax, the HR value was significantly increased (p<0.05) as shown in Table 2.
| Group | +dp/dtmax (mmHg/s) | -dp/dtmax (mmHg/s) | HR (times/min) | LVESP (mmHg) | LVEDP (mmHg) |
|---|---|---|---|---|---|
| Sham surgery | 5489.11±143.56 | 5281.94±141.70 | 385.54±50.32 | 122.01±16.41 | 2.25±0.46 |
| Model set | 3244.16±114.68a | 2701.64±105.95a | 255.42±21.29a | 76.46±11.03a | 5.61±1.33a |
| Liraglutide | 4675.93±135.09b | 4831.68±127.09b | 336.49±36.07b | 105.80±13.58b | 3.03±0.77b |
| LY2940002 | 2750.37±106.11c | 2019.26±118.06c | 205.29±22.50c | 64.83±10.22c | 7.11±1.42c |
| Liraglutide+LY2940002 | 3930.62±128.40cd | 3518.49±120.56cd | 289.38±33.23cd | 90.23±14.35cd | 4.21±1.17cd |
Note: When compared to the sham surgery group, ap<0.05; compared to the model group, bp<0.05; compared to the liraglutide group, cp<0.05 and compared with the LY2940002 group, dp<0.05
Table 2: Comparison of hemodynamic indexes of rats in each group (X̄±S, N=12)
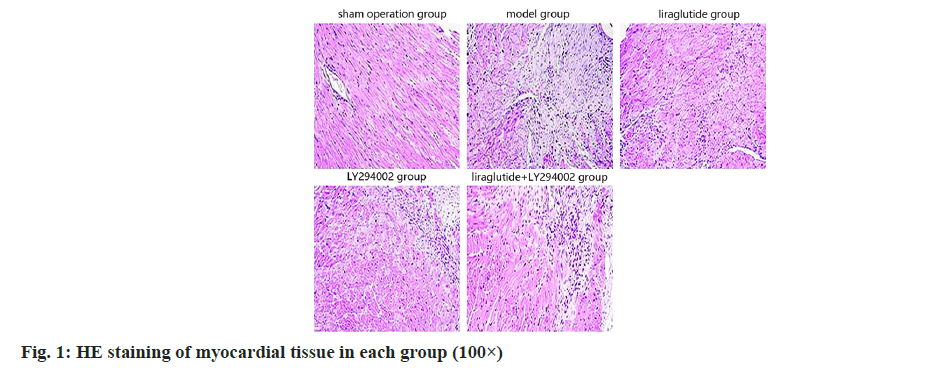
The myocardial tissue and morphology of the rats were almost normal, and the necrosis of the myocardial tissue damage of the rats was significantly decreased as shown in fig. 1.
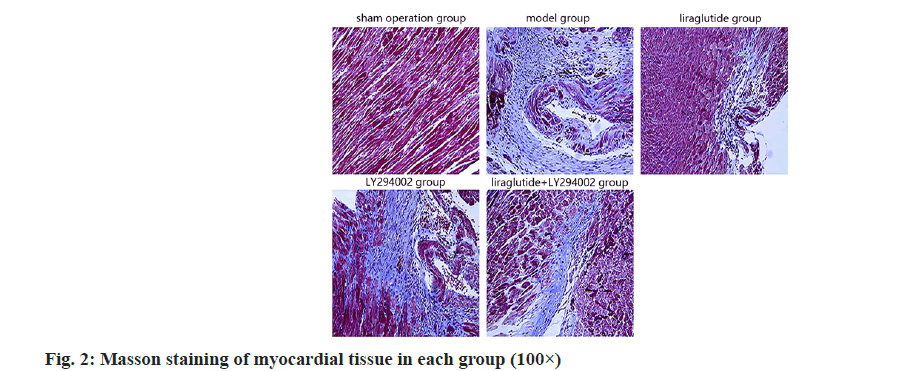
In the sham group, myocardial tissues were clear and muscle fibers were normal; in the model group, collagen fibers were increased and myocardial fibrosis was decreased; in the liraglutide group; compared with the LY294002 group as shown in fig. 2.
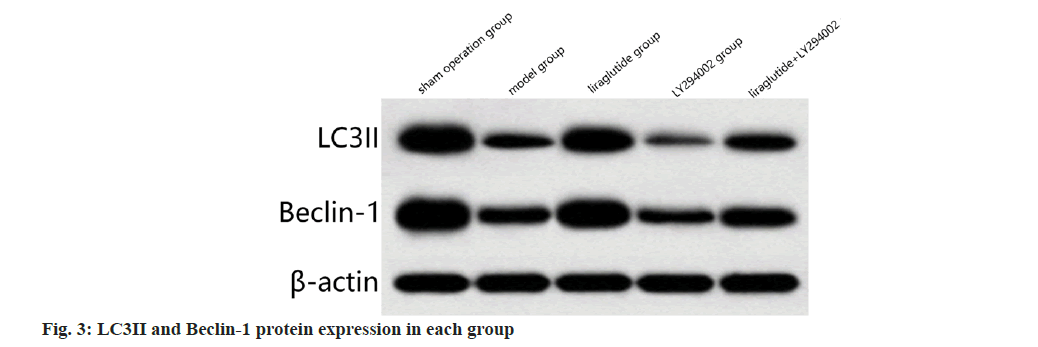
Compared to the sham surgery group, the protein expression of LC3II and Beclin-1 was significantly decreased in the model group (p<0.05); compared to the model group, LC3II and Beclin-1 protein expression were increased significantly in liraglutide group (p<0.05); in contrast to the liraglutide group, LC3II and Beclin-1 protein expression was significantly decreased in liraglutide+LY2940002 group (p<0.05); compared with the LY294002 group, liraglutide+LY2940002 group, the LC3II and Beclin-1 protein expression was significantly increased (p<0.05) as shown in fig. 3 and Table 3.
| Group | LC3II/β-actin | LC3II/β-actin |
|---|---|---|
| Sham surgery | 1.23±0.18 | 1.48±0.10 |
| Model set | 0.21±0.03a | 0.29±0.12a |
| Liraglutide | 0.84±0.09b | 1.14±0.08b |
| LY2940002 | 0.08±0.03c | 0.12±0.04c |
| Liraglutide+LY2940002 | 0.35±0.07cd | 0.41±0.07cd |
Note: When compared to the sham surgery group, ap<0.05; compared to the model group, bp<0.05; compared to the liraglutide group, cp<0.05 and compared with the LY2940002 group, dp<0.05
Table 3: Comparison of LC3II and Beclin-1 protein expression in myocardial Tissue of each group (X̄±S, N=12)
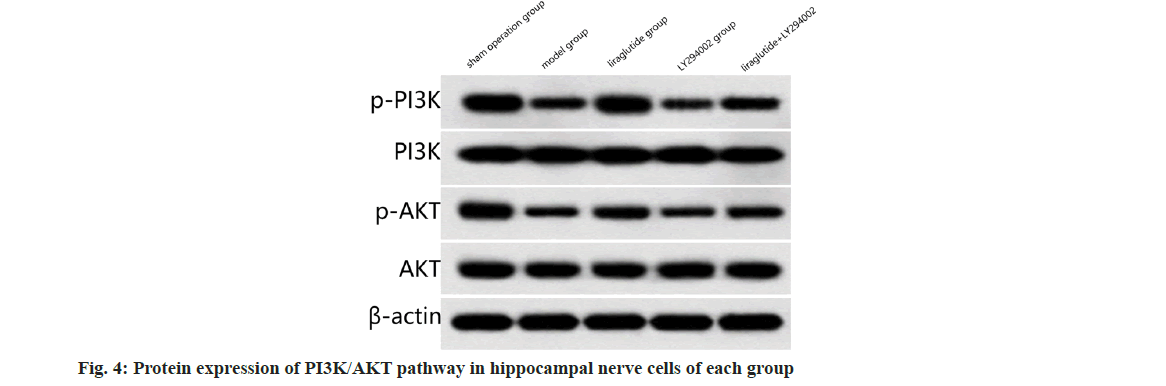
Compared to the sham surgery group, PI3K/ AKT pathway (p<0.05); compared to the model group, the expression of PI3K/AKT pathway was significantly increased in liraglutide group (p<0.05); in contrast to the liraglutide group, the expression of PI3K/AKT pathway was significantly decreased in liraglutide+LY2940002 group (p<0.05); compared with the LY2940002 group, the PI3K/AKT pathway was significantly increased in liraglutide+LY2940002 group (p<0.05) as shown in fig. 4 and Table 4.
| Group | p-PI3K/PI3K | p-AKT/AKT |
|---|---|---|
| Sham surgery | 1.29±0.20 | 1.31±0.30 |
| Model set | 0.37±0.07a | 0.41±0.08a |
| Liraglutide | 0.89±0.18b | 0.92±0.19b |
| LY2940002 | 0.20±0.04c | 0.33±0.06c |
| Liraglutide+LY2940002 | 0.59±0.13cd | 0.53±0.14cd |
Note: When compared to the sham surgery group, ap<0.05; compared to the model group, bp<0.05; compared to the liraglutide group, cp<0.05 and compared with the LY2940002 group, dp<0.05
Table 4: Comparison of PI3K-AKT Pathway Protein expression in all rats (X̄±S, N=12)
In recent years, the incidence of AMI has been increasing, and the mortality and disability rate are very high, which has become one of the main causes of heart failure. The pathogenesis of AMI is complex and related to coronary atherosclerotic plaque rupture, platelet aggregation, thrombosis, persistent coronary artery occlusion and myocardial ischemia/hypoxia necrosis[13,14]. AMI often leads to abnormal cardiac function, myocardial necrosis, myocardial tissue inflammation and fibrosis, and eventually causes heart failure, arrhythmia and other adverse symptoms, posing a threat to the life safety of patients[15], therefore, it is important to establish corresponding AMI animal models to study AMI. In the study, AMI rat model was often used to construct by coronary artery ligation. The established animal model was relatively stable and similar to the pathophysiological process of human AMI[16]. The results of this study showed that the serum levels of CK-MB, cTnI and LVEDP value of AMI rats were significantly increased, the indicators of cardiac function were significantly reduced, a large number of inflammatory cell infiltration, cell necrosis, muscle fiber disorder, and the degree of fibrosis of myocardial tissue were seen with Huifang the results were similar, indicating that AMI can cause increased myocardial fibrosis and lead to abnormal cardiac function, indicating that the construction of AMI rat model was successful.
Elevated blood glucose is a common metabolic disorder in AMI patients. As a GLP-1 analogue, liraglutide is an insulin hormone that regulates blood glucose and has anti-oxidation and antiinflammatory properties that protect against endothelial function[17]. Previous studies have found that GLP-1R activators can directly activate the signaling pathways in cardiomyocytes and improve cardiac injury[18]. In this study, after AMI mice were treated with liraglutide, it was found that various cardiac function indexes and blood rheological indexes gradually recovered, and the degree of myocardial tissue damage and fibrosis were improved. We speculate that liraglutide could significantly improve the impaired cardiac function caused by AMI.
Autophagy is a highly conserved self-degradation process with multiple connections to human diseases because its function is critical for cellular stress response, dynamic balance, survival, and organismal development[19]. Recent studies suggest that persistent cardiac ischemia impairs autophagy in cardiomyocytes, thereby exacerbating unfavorable cardiac remodeling after AMI[20,21]. The results of this study found that the expression of autophagy-related proteins (LC3II and Beclin-1) was significantly downregulated in the myocardial tissue of AMI mice, while the expression changes were significantly reversed after liraglutide administration. Our results indicate that liraglutide activates the autophagy response in AMI to promote cardiac remodeling and improve myocardial injury.
The PI3K/AKT signaling pathway has antiapoptotic and autophagy-regulating effects in heart disease[22]. PI3K/AKT pathway plays an important role in cell growth, proliferation, migration, activated PI3K can activate AKT, activated AKT can promote the expression of anti-apoptotic protein, inhibit the generation of proapoptotic protein, and promote the growth of vascular endothelial cells, and reduce the cardiac cell apoptosis, promote myocardial tissue angiogenesis, play a myocardial protective effect[23]. The results of this study showed that liraglutide promotes the expression of these proteins in the PI3K/AKT/mammalian Target of Rapamycin (mTOR) pathway, indicating that liraglutide activates the PI3K/AKT pathway. Compared with liraglutide group, PI3K/AKT pathway related protein expression was significantly decreased in liraglutide+LY2940002 rats, and significantly higher in liraglutide+LY2940002 rats, suggesting that the function of liraglutide in protecting myocardial injury may be achieved through activation of the PI3K/AKT pathway.
In conclusion, liraglutide could improve myocardial injury and protect cardiac function by activating the PI3K/AKT signaling pathway. This study only preliminarily explored the effect of liraglutide on AMI by regulating PI3K/AKT signaling pathway. However, it is still unclear whether liraglutide acts on AMI treatment through other pathways, so further research is still needed.
Author’s contributions:
Yu Lu and Houxiang Qin have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Chan MY, Koh KW, Poh SC, Marchesseau S, Singh D, Han Y, et al. Remote postdischarge treatment of patients with acute myocardial infarction by allied health care practitioners vs. standard care: The IMMACULATE randomized clinical trial. JAMA Cardiol 2021;6(7):830-5.
[Crossref] [Google Scholar] [PubMed]
- Faresjo T, Stromberg S, Jones M, Stomby A, Karlsson JE, Ostgren CJ, et al. Elevated levels of cortisol in hair precede acute myocardial infarction. Sci Rep 2020;10(1):22456.
[Crossref] [Google Scholar] [PubMed]
- Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017;136(9):849-70.
[Crossref] [Google Scholar] [PubMed]
- Kajiwara M, Tanaka A, Kawasaki T, Nakao K, Sakamoto T, Toyoda S, et al. Safety and efficacy of liraglutide treatment in Japanese type 2 diabetes patients after acute myocardial infarction: A non-randomized interventional pilot trial. J Cardiol 2017;69(3):511-7.
[Crossref] [Google Scholar] [PubMed]
- Kyhl K, Lønborg J, Hartmann B, Kissow H, Poulsen SS, El Ali H, et al. Lack of effect of prolonged treatment with liraglutide on cardiac remodeling in rats after acute myocardial infarction. Peptides 2017;93:1-2.
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Huang DD, Li DG, Chen B, Zhang LM, Yuan CL, et al. Tetramethylpyrazine exerts a protective effect against injury from acute myocardial ischemia by regulating the PI3K/Akt/GSK-3β signaling pathway. Cell Mol Biol Lett 2019;24(1):1-2.
- Cheng S, Zhang X, Feng Q, Chen J, Shen L, Yu P, et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci 2019;227:82-93.
[Crossref] [Google Scholar] [PubMed]
- Meng H, Zhang Y, An ST, Chen Y. Annexin A3 gene silencing promotes myocardial cell repair through activation of the PI3K/Akt signaling pathway in rats with acute myocardial infarction. J Cell Physiol 2019;234(7):10535-46.
[Crossref] [Google Scholar] [PubMed]
- Zeng SS, Bai JJ, Jiang H, Zhu JJ, Fu CC, He MZ, et al. Treatment with liraglutide exerts neuroprotection after hypoxic–ischemic brain injury in neonatal rats via the PI3K/AKT/GSK3β pathway. Front Cell Neurosci 2020;13:585.
[Crossref] [Google Scholar] [PubMed]
- Li C, Zhang Y, Wang Q, Meng H, Zhang Q, Wu Y, et al. Dragon's blood exerts cardio-protection against myocardial injury through PI3K-AKT-mTOR signaling pathway in acute myocardial infarction mice model. J Ethnopharmacol 2018;227:279-89.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhou Y, Li J. Studies on the effects of liraglutide in osteoporosis rats with type 2 diabetes based on the PI3K/Akt pathway. Chin J Osteoporos 2021;27(7):985-9.
- Xu G, Fu J, Zhao X, Wang X. Role of PI3K/Akt/Nrf 2 signaling in the protective effects of resveratrol pretreatment on myocardial in diabetic rats. Chin J Anesthesiol 2019;10:1189-93.
- Troester V, Strebel I, Nestelberger T, Boeddinghaus J, Rubini Gimenez M, Lopez-Ayala P, et al. Association of previous myocardial infarction and time to presentation with suspected acute myocardial infarction. J Am Heart Assoc 2021;10(1):e017829.
[Crossref] [Google Scholar] [PubMed]
- Yi M, Li H, Wang X, Yan J, Gao L, He Y, et al. Ion therapy: A novel strategy for acute myocardial infarction. Adv Sci 2019;6(1):1801260.
- Alvino VV, Fernández-Jiménez R, Rodriguez-Arabaolaza I, Slater S, Mangialardi G, Avolio E, et al. Transplantation of allogeneic pericytes improves myocardial vascularization and reduces interstitial fibrosis in a swine model of reperfused acute myocardial infarction. J Am Heart Assoc 2018;7(2):e006727.
[Crossref] [Google Scholar] [PubMed]
- Lu H, Fang Y, Li Y. Effects and mechanisms of liraglutide on angiogenesis and myocardial protection in rats with acute myocardial infarction. Chin Circ Magazine 2017;32(11):1117-22.
- Chen WR, Tian F, Dai Chen Y, Wang J, Yang JJ, Wang ZF, et al. Effects of liraglutide on no-reflow in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol 2016;208:109-14.
[Crossref] [Google Scholar] [PubMed]
- Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009;58(4):975-83.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Klionsky DJ. Autophagy and disease: Unanswered questions. Cell Death Differ 2020;27(3):858-71.
[Crossref] [Google Scholar] [PubMed]
- Wu X, He L, Chen F, He X, Cai Y, Zhang G, et al. Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS One 2014;9(11):e112891.
[Crossref] [Google Scholar] [PubMed]
- Wu X, Zheng D, Qin Y, Liu Z, Zhang G, Zhu X, et al. Nobiletin attenuates adverse cardiac remodeling after acute myocardial infarction in rats via restoring autophagy flux. Biochem Biophys Res Commun 2017;492(2):262-8.
[Crossref] [Google Scholar] [PubMed]
- Xuan F, Jian J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int J Mol Med 2016;38(1):328-36.
[Crossref] [Google Scholar] [PubMed]
- Zheng LZ. Effect of Ligusticum canadense soup on PI3K/Akt signal transduction pathway in myocardial infarction rats. J Tradit Chin Med 2019;47(3):19-23.