- *Corresponding Author:
- Jiangyan Zhou
Department of Gynecology, Jiangxi Maternal and Child Health Hospital, Nanchang, Jiangxi 330000, China
E-mail: zjyxinjian@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “27-35” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To survey the effects of hysteroscopic surgery combined with hormone monitoring and hormone therapy on postoperative symptom improvement and prognosis of patients with functional uterine bleeding, and to analyse its safety. From November 2021 to March 2023, 94 patients with functional uterine bleeding treated in our hospital were arbitrarily assigned into observation group (n=47) and control group (n=47). On both groups, hysteroscopic endometrial resections were performed. Hormone monitoring and hormone therapy were given to the observation group, while no additional treatment was given to the control group. The therapeutic effects of both groups were carefully observed, including the time taken for clinical symptom improvement and the incidence of adverse reactions. Additionally, a comparison was made in terms of sex hormone levels, uterine-related parameters, serum indexes, and quality of life scores before and after treatment. After treatment, the levels of angiopoietin-2 and aquaporin 4 in both groups were lessened, and vascular endothelial growth factor was associated with the endothelial growth factor. After hysteroscopic surgery, hormone monitoring and hormone therapy have remarkable therapeutic effects on patients with functional uterine bleeding, which can remarkably lessen the time of clinical symptom improvement, regulate the level of sex hormones, improve serum indexes, and have few adverse reactions. This treatment program is worthy of clinical promotion.
Keywords
Hysteroscopic surgery, Hormone therapy, unclassified bleeding disorders, prognosis
Functional uterine hemorrhage, alternatively referred to as Bleeding of Unknown Cause (BUC), are characterized by a noticeable propensity to bleed despite the absence of abnormalities in standard hemostatic tests. A patient’s quality of life is adversely affected by functional uterine hemorrhage. The main reason is that the decline of ovarian function leads to the disorder of reproductive function and autonomic nerve function, which leads to functional bleeding, which leads to serious anemia in different patients, and even needs hysterectomy. Make the patient completely lose fertility[1,2]. At present, the most used method for the treatment of dysfunctional uterine bleeding is Western medicine, but the treatment of synthetic drugs has some adverse reactions and side effects[3,4]. At the same time, prolonged bleeding may result in chronic anemia, which can increase medical costs and place a remarkable burden on families and the community[5].
In the past, patients with functional uterine bleeding without fertility need were usually cured with total hysterectomy, but several studies have shown that conservative treatment is not effective[6]. Finally, patients may choose hysterectomy due to treatment failure or life-threatening massive blood loss[7,8]. There are many factors that prevent patients from undergoing total hysterectomy, such as coagulation abnormalities, history of multiple abdominal surgeries, pathologic obesity, and inability to tolerate total hysterectomy in patients with severe cardiovascular complications[9,10]. It is therefore extremely important to find a surgical method that preserves the uterus while achieving therapeutic effectiveness.
The first generation of Trans Cervical Resection of Endometrium (TCRE) removes the whole layer of endometrium and part of myometrium under it through the action of electric current, and prevents endometrial regeneration to achieve the effect of amenorrhea[11]. In comparison with hysterectomy, it causes less trauma, takes less time, and has no effect on ovarian functions. During the past few years, it has been widely used in China for patients with abnormal uterine bleeding without a need for reproduction[12,13]. There is some risk of recurrence after TCRE, and some patients will have a second surgery to remove the uterus because of renewed vaginal bleeding. Different complications can have an impact on recurrence after TCRE. Therefore, new and effective treatment programs are important to better improve the surgical outcome and reduce the recurrence rate after TCRE.
The postoperative Progesterone (P) therapy can remarkably reduce disease recurrence[14]. Although there were many studies on the effect of hormone therapy on functional uterine hemorrhage there were great differences in experimental design, observation index and sample acceptance standard. In this context, it is necessary to continue to carry out indepth research to draw more scientific conclusions and lay a theoretical foundation for the popularization and application of hormone therapy when treating patients with functional uterine hemorrhage.
Materials and Methods
General information:
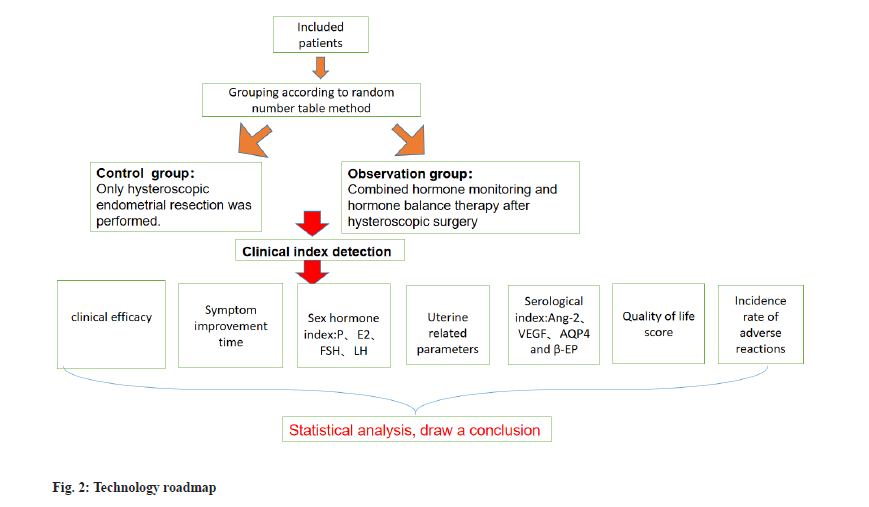
From November 2021 to March 2023, a total of 94 patients with functional uterine hemorrhage were enrolled as participants in our hospital. These individuals were arbitrarily assigned into two groups: The observation group and the control group, each comprising 47 cases. Both groups underwent hysteroscopic endometrial resection. The observation group was given hormone monitoring and hormone therapy after operation, while the control group was not given other treatments after operation.
The observation group was composed of individuals aged between 38 y and 59 y, with an average age of (48.04±11.21) y. The duration of the disease varied from 3 mo to 3 y, with an average duration of (1.73±0.82) y. Among the participants, 3 were unmarried, while 44 were married. The average number of pregnancies was (2.05±0.56). The Body Mass Index (BMI) ranged from 17.22 to 27.69 kg/ m2, with an average of (22.69±2.10) kg/m2. Similarly, the control group consisted of individuals aged 39 y to 60 y, with an average age of (47.99±10.78) y. The duration of the disease ranged from 5 mo to 4 y, with an average duration of (1.76±0.75) y. Among the participants, 2 were unmarried, while 45 were married. The average number of pregnancies was (2.08±0.58). The BMI ranged from 17.16 to 27.71 kg/m2, with an average of (22.58±2.12) kg/m2. No remarkable difference was found in age, course of disease and other general data (p>0.05). An informed consent form was signed by each patient, and the study was approved by the medical ethics committee of the sample hospital.
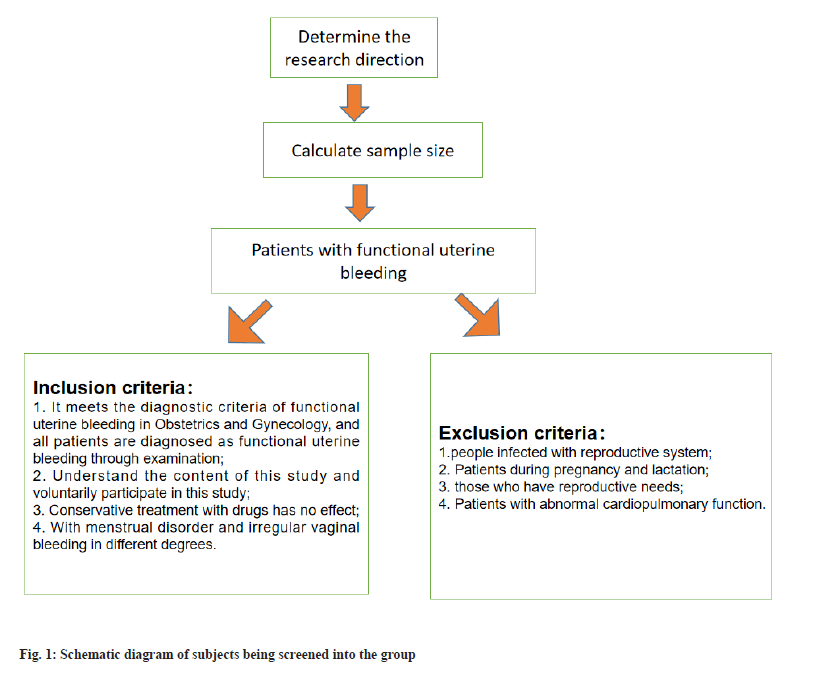
Inclusion criteria: According to the relevant diagnostic criteria of functional uterine bleeding in obstetrics and gynecology[15], and all the patients were diagnosed as functional uterine bleeding by examination; the patient participants understood the contents of the study and volunteered to participate; there was no effect of conservative drug treatment and all the patients were accompanied with different degrees of menstrual disorder and irregular vaginal bleeding.
Exclusion criteria: Patients with reproductive system infection; patients during pregnancy and lactation; patients with reproductive needs; patients with abnormal cardiopulmonary function and patients with mental diseases and cognitive impairment.
Calculation formula of sample size:
Bilateral Alpha (α) was 0.05, Beta (β) was 0.20, and the clinical efficacy (total effective rate) was taken as the effect index to consult the relevant literature and previous studies Ma Aili, Clinical effect of sex hormone when treating adolescent functional uterine bleeding. China Health Nutrition, 2020, 30 (25):75, p1=0.95, p2=0.75. After calculation, the sample size of each group was 42 cases, and based on the shedding rate of 10 %, there were about 47 patients in each group, with 94 patients (fig. 1).
Treatment methods:
All patients improved the relevant examinations before operation, fasting 12 h before operation and drinking 6 h before operation. The patients in both groups underwent hysteroscopic endometrial resection. The patients took the lithotomy position, underwent intravenous general anesthesia, applied 0.9 % Sodium chloride (Nacl) solution as uterine distention fluid, and then underwent hysteroscopic endometrial resection and electrocoagulation to stop bleeding. During the procedure, damage to the patient’s myometrium was avoided, including the uterine fundus, the anterior, posterior, and lateral uterine walls up to 5 mm from the cervical opening. The bleeding spots and oozing blood in the uterine cavity of the patients were observed after operation. The observation group combined with hormone monitoring and hormone therapy, including medroxyprogesterone acetate (Zhejiang Xianzheng Pharmaceutical Co., Ltd. National Medicine Zhunzi H33020715, 2 mg*100 s) was given orally, with a single dose of 4 mg twice a day. Estradiol (E2) valerate (Bayer Pharmaceutical and Health Co., Ltd. Guangzhou Branch, J20130006) were given. The usage amount of E2 valerate was Sig 4 mg, p.o, q. 6~8 h. After hemostasis, the dose was adjusted to 2 mg/d, and the administration was stopped 20 d after hemostasis. The control group did not receive any hormone therapy after operation.
Observation index:
Evaluation standard of curative effect[16]: Remarkable effect means that the menstrual performance of the patient returned to normal after treatment; effective means that the menstrual performance of the patient improved remarkably after treatment and ineffective means that the menstrual performance of the patient did not change after treatment. Total effective rate=(number of extremely effective patients+number of effective patients)/ total number of patients×100 %. The patients in the observation group were assessed after treatment, specifically 20 d after hemostasis and the completion of treatment. On the other hand, the patients in the control group were evaluated 1 mo after the surgical procedure.
The time of symptom improvement: The improvement time of symptoms such as dripping, sore waist and knees, abdominal pain, dysmenorrhea, and light menstruation were compared.
Sex hormone levels: The peripheral venous blood 7 ml of the patient was centrifuged with velocity 3000 r/min and radius 0.5 cm, and the serum was taken to detect the sex hormone index by Enzyme-Linked Immunosorbent Assay (ELISA), including P, E2, Follicle-Stimulating Hormone (FSH), and Luteinizing Hormone (LH). The testing equipment was Mindray BS-280 automatic biochemical analyzer provided by Nanjing Beidan Medical Co., Ltd. and the kit was provided by Shanghai Enzymatic Biotechnology Co., Ltd. The levels of sex hormones were assessed both before treatment (prior to intervention) and after treatment (30 d post-surgery) in both groups.
Uterine related parameters: Before treatment (before treatment) and 30 d after treatment (30 d after treatment), the endometrial thickness, Pulse Index (PI) and Resistance Index (RI) of uterine artery were detected by Minui DC-N2S color Doppler ultrasound provided by Nanjing Bayden Medical Co., Ltd. and the results were determined and recorded by two radiologists.
Serological indexes: Angiopoietin-2 (Ang-2) and Vascular Endothelial Growth Factor (VEGF) were measured by ELISA (DG5033A) before (preoperative) and 30 d after (postoperative) treatment in both groups. VEGF, Aquaporin 4 (AQP4) and β-Endorphin (β-EP) levels were all purchased from Shanghai Future Industrial Co., Ltd.
A number of factors were evaluated when evaluating life quality, including daily life function, psychological function, social function, and material function[17]. Before treatment and 30 d after operation, patients were evaluated.
Adverse drug reactions: Within 1 mo after surgery, the incidence of nausea, vomiting, anorexia, general weakness, and drug rash was compared. The total incidence of adverse reactions=the sum of all kinds of adverse reactions/the total number of cases×100 %. Nausea: A discomfort in the stomach that can cause vomiting impulses; vomiting: Stomach contents are forcefully excreted through the mouth; anorexia: Patient’s desire to eat decreases in varying degrees, and even anorexia can occur in severe cases; general weakness: The feeling of fatigue and weakness in the body and drug rash: An inflammation on the surface of the skin, a general term for various signs of skin damage (fig. 2).
Statistical analysis:
Analysis and processing of data were carried out using Statistical Package for the Social Sciences (SPSS) 22.0. A (x̄ ±s) symbol is used to indicate measurements with a normal distribution or approximate normal distribution. Comparing the two groups was done using paired t-tests, while comparing the two groups separately using independent sample t-tests. The n (%) was adopted to represent the counting data, and χ2 test was adopted. p<0.05 was the differences were statistically remarkable.
Results and Discussion
Treatment effectiveness was 95.74 % in the observation group, which was remarkably higher than 76.60 % in the control group (p<0.05) as shown in Table 1.
| Group | n | Remarkable effect | Effective | Invalid | Total efficacy |
|---|---|---|---|---|---|
| Observation | 47 | 38 (80.85) | 7 (14.89) | 2 (4.44) | 45 (95.74) |
| Control | 47 | 23 (51.11) | 13 (27.66) | 11 (23.40) | 36 (76.60) |
| χ2 | 7.231 | ||||
| p | <0.05 |
Table 1: Comparison of Clinical Efficacy (n %)
The improvement time of clinical symptoms such as dripping, sore waist and knees, abdominal pain, dysmenorrhea, and light menstruation in the observation group were remarkably shorter (p<0.05) as shown in Table 2.
| Group | n | Dripping and dripping | Sore waist and knees | Abdominal pain | Menstrual irregularities | Light menstrual blood color |
|---|---|---|---|---|---|---|
| Observation | 47 | 2.09±0.25 | 2.21±0.23 | 2.19±0.22 | 2.38±0.09 | 2.23±0.15 |
| Control | 47 | 4.78±0.31 | 4.87±0.45 | 4.93±0.19 | 4.62±0.21 | 4.72±0.18 |
| t | 46.307 | 36.084 | 64.621 | 67.214 | 72.855 | |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Table 2: Comparison of Clinical Symptom Improvement Time (x̄±s, days)
Before treatment, no remarkable difference was found in serum P, E2, FSH and LH levels (p>0.05). Following treatment, there was a remarkable reduction in the serum levels of P, E2, FSH, and LH in both groups. Notably, the observed improvement effect in the observation group was notably more pronounced (p<0.05) as shown in Table 3.
| Group | p (mmol/l) | E2 (pmol/ml) | FSH (U/l) | LH (mIU/ml) | ||||
|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Observation | 5.41±1.38 | 2.03±0.29a | 153.23±10.04 | 94.42±5.62a | 122.51±10.04 | 81.04±5.21a | 82.47±10.14 | 41.19±5.33a |
| Control | 5.22±1.29 | 2.94±0.63b | 152.98±9.85 | 107.72±5.49b | 122.71±10.32 | 90.03±5.62b | 81.94±10.09 | 48.82±5.66b |
| t | 0.69 | 8.995 | 0.122 | 11.606 | 0.095 | 8.042 | 0.254 | 6.728 |
| p | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 |
Note: Compared with the observation group before treatment, ap<0.05 and compared with the control group before treatment, bp<0.05
Table 3: Comparison of Sex Hormone Levels before and after Treatment (X̄±S)
Before treatment, no remarkable difference was found in endometrial thickness, uterine artery PI and uterine artery RI (p>0.05). After treatment, the endometrial thickness, PI and RI of uterine artery parameters in the observation group were remarkably lower (p<0.05) as shown in Table 4.
| Group | Endometrial thickness (mm) | PI | RI | |||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Observation | 8.22±2.44 | 3.12±0.41a | 1.89±0.12 | 1.01±0.18a | 1.42±0.14 | 0.38±0.08a |
| Control | 8.36±2.28 | 5.56±0.83b | 1.85±0.13 | 1.53±0.23b | 1.45±0.22 | 1.14±0.12b |
| t | 0.287 | 10.07 | 1.55 | 12.206 | 0.789 | 36.127 |
| p | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 |
Note: Compared with the observation group before treatment, ap<0.05 and compared with the control group before treatment, bp<0.05
Table 4: Comparison of Uterine Related Parameters before and after Treatment (x̄±s)
Before treatment, the levels of Ang-2, VEGF, AQP4 and β-EP were compared, and no statistical difference was found (p>0.05). Following treatment, the levels of Ang-2 and AQP4 in both groups lessened, whereas the levels of VEGF and β-EP elevated. Notably, the observation group demonstrated a remarkably better improvement effect (p<0.05) as shown in Table 5.
| Group | Ang-2 (ng/l) | VEGF (ng/l) | AQP4 (ng/l) | β-EP (ng/l) | ||||
|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Observation | 284.45±12.42 | 194.58±9.76a | 2433.83±66.72 | 3124.42±73.32a | 5.73±0.23 | 3.04±0.12a | 112.31±9.42 | 134.81±12.42a |
| Control | 284.71±12.51 | 227.45±9.84b | 2432.91±67.21 | 2781.13±71.06b | 5.81±0.22 | 4.72±0.11b | 111.94±9.45 | 121.15±12.37b |
| t | 0.101 | 16.259 | 0.067 | 23.05 | 1.723 | 70.751 | 0.195 | 5.342 |
| p | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 |
Note: compared with the observation group before treatment, ap<0.05 and compared with the control group before treatment, bp<0.05
Table 5: Comparison of Serum Indexes before and after Treatment (Points, x̄±s)
Before treatment, no remarkable difference was found in the scores of daily life function, psychological function, social function, and material function (p>0.05). Post-treatment, the scores reflecting various dimensions of patient’s quality of life in both groups revealed a remarkable improvement. Furthermore, the observation group exhibited a superior enhancement in quality of life (p<0.05, Table 6).
| Group | Daily life function | Psychological function | Social function | Material function | ||||
|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Observation | 32.01±0.25 | 59.14±9.83a | 21.71±2.04 | 67.91±8.48a | 30.31±0.04 | 66.78±7.21a | 22.41±8.52 | 69.34±8.17a |
| Control | 31.94±0.28 | 47.76±7.81b | 21.65±2.11 | 56.73±9.04b | 30.32±0.05 | 52.31±4.62b | 22.73±8.01 | 55.67±2.83b |
| t | 1.278 | 6.214 | 0.14 | 6.184 | 1.071 | 11.585 | 0.188 | 10.839 |
| p | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 |
Note: Compared with the observation group before treatment, ap<0.05 and compared with the control group before treatment, bp<0.05
Table 6: Comparison of Life Quality Scores (Points, x̄±s)
A remarkably low incidence of adverse reactions was observed in the observation group, 4.26 %, compared to 27.66 % in the control group (p<0.05) as shown in Table 7.
| Group | n | Nausea and vomiting | Anorexia | Weakness | Drug rash | Total incidence rate (%) |
|---|---|---|---|---|---|---|
| Observation | 47 | 1 (2.13) | 0 (0.00) | 0 (0.00) | 1 (2.13) | 2 (4.26) |
| Control | 47 | 3 (6.38) | 2 (4.26) | 4 (8.51) | 4 (8.51) | 13 (27.66) |
| χ2 | 9.598 | |||||
| p | <0.05 |
Table 7: Comparison of Adverse Reactions (n/%)
With the increase of age, women will not be able to stop menstruation due to the decline or disappearance of ovarian function. At present, this period is called menopause in clinic. When the ovaries of menopausal women lose the function of promoting ovulation, the level of sex hormones in their bodies will no longer change periodically, and some women will have irregular uterine bleeding during this period, which is clinically referred to as menopausal functional uterine bleeding[18,19]. Functional uterine bleeding is common in clinic. If women confirm irregular vaginal bleeding, after excluding tumor, inflammation, trauma and other factors, they can be diagnosed as menopausal functional uterine bleeding when intrauterine devices are not available[20]. Based on the pathogenesis of menopausal functional uterine bleeding, P supplementation will be given priority in clinical treatment, and corresponding blood cell supplementation will be given according to the patient’s bleeding situation. In addition, necessary nutritional support and psychological support are also the key to improve the symptoms of such patients and control the deterioration of their condition[21].
TCRE removes the functional layer of the endometrium, the whole layer of the basal layer and part of the muscular layer below it by electro resection, prevents endometrial regeneration to achieve the effect of amenorrhea or reduce menstruation, and controls excessive bleeding[22,23]. Hysteroscopic endometrial electro surgery is widely acknowledged and favored by a significant number of patients due to its numerous advantages, including minimal trauma, reduced procedure duration, faster postoperative recuperation, and notable therapeutic effectiveness. Surgical treatment of patients with abnormal uterine bleeding with TCRE resulted in improvement of symptoms and remarkable reduction of menstrual flow in most patients. However, there are some patients whose symptoms do not improve remarkably within a short period of time and require hormonal therapy after surgery, which may affect the patient’s satisfaction with the outcome of the treatment. Many patients find it difficult to accept that their symptoms have only slightly improved and ultimately opt for hysterectomy[24]. To enhance the long-term prognosis of patients, better surgical techniques are needed to ensure complete resection of the endothelium. Combining this with other treatments to minimize postoperative recurrence rates is of great practical importance.
The results of this study showed that treatment effectiveness was 95.74 % in the observation group, which was remarkably higher than 76.60 % in the control group the improvement time of clinical symptoms such as dripping, sore waist and knees, abdominal pain, dysmenorrhea and light menstruation in the observation group were remarkably shorter. After treatment, the levels of PI and RI in uterine artery, serum P, E2, FSH and LH in both groups were remarkably lessened, and the thickness of endometrium was also lessened, and the improvement effect in the observation group was remarkably better. These results have shown that the combination of hormone monitoring and hormone therapy after hysteroscopic surgery has a remarkable therapeutic effect on functional uterine bleeding, which can quickly improve the bleeding symptoms and endometrial morphology of patients and adjust the level of sex hormones. Sex hormones have an important physiological function in the endocrine system of human body. Due to abnormalities in ovarian function and endometrial morphology, menopausal patients with functional uterine bleeding will experience a marked increase in the levels of various hormones in their bodies. The remaining ovaries in the ovary will continue to grow and develop, but their ovulatory function has been completely lost. When sex hormone levels fluctuate remarkably, the proliferative response and incomplete shedding of the endometrial glands can lead to irregular vaginal bleeding[25,26]. In this study, postoperative hormone monitoring and hormone therapy in the observation group can effectively regulate the hormone level in patients and enhance the symptoms and physiological indexes of patients.
Ang-2 attaches importance to maintaining the stability of vascular intima. It is highly expressed in patients with functional uterine bleeding[27]. VEGF is an important angiogenic factor, and its elevated expression can promote endometrial repair[28]. AQP4 is an aquaporin that increases endometrial vascular permeability, resulting in interstitial edema, humoral circulation disturbance and functional uterine bleeding[29]. β-EP is a type of endogenous opioid peptide found in the hypothalamic arcuate nucleus and pituitary gland, which exerts remarkable influence over the regulation of female reproductive endocrine activities[30]. After treatment, the serum levels of Ang- 2, VEGF, AQP4 and β-EP were remarkably improved in this study, especially in the treatment group, suggesting that hormone monitoring and hormone therapy after hysteroscopy can obviously improve the level of cytokines in the body. In this study, the enhancement of various quality of life scores of patients in the observation group was remarkably better, and the incidence of adverse reactions was lower, suggesting that the combination of hormone monitoring and hormone therapy after hysteroscopy has a reliable therapeutic effect and high safety on patients with functional uterine bleeding. After postoperative hormone monitoring and hormone therapy, the patient’s conditions were remarkably improved, their psychological burden was reduced, and they were able to return to normal life as soon as possible. A low incidence of adverse reactions was observed in the observation group because the therapeutic drugs used were mild and could be tolerated by the patients, while the adverse reactions brought about by the surgical treatment could also be alleviated.
In conclusion, compared with hysteroscopic surgery alone, hormone monitoring and hormone therapy can play an obvious synergistic effect on patients with postoperative functional uterine bleeding, which can remarkably shorten the time of clinical symptom improvement, regulate the hormone level, and improve uterine parameters and serum indexes while the treatment safety is higher, and the patient’s life quality is remarkably improved. As a result, regional differences and feedback were not documented in this study due to a small sample size. It is suggested that cross-regional, multi-center, and large-sample studies can be conducted in future studies to obtain more accurate evidence and better serve the clinic.
Conflict of interests:
The authors declared no conflict of interests.
References
- Yaşa C, Uğurlucan FG. Approach to abnormal uterine bleeding in adolescents. J Clin Res Pediatr Endocrinol 2020;12(1):1.
[Crossref] [Google Scholar] [PubMed]
- Critchley HO, Maybin JA, Armstrong GM, Williams AR. Physiology of the endometrium and regulation of menstruation. Physiol Rev 2020;100(3):1149-79.
- Donnez J, Carmona F, Maitrot-Mantelet L, Dolmans MM, Chapron C. Uterine disorders and iron deficiency anemia. Fertil Steril 2022 ;118(4):615-24.
[Crossref] [Google Scholar] [PubMed]
- Serres-Cousine O, Kuijper FM, Curis E, Atashroo D. Clinical investigation of fertility after uterine artery embolization. Am J Obstet Gynecol 2021;225(4):403-e1.
[Crossref] [Google Scholar] [PubMed]
- Franke D, Zepf J, Burkhardt T, Stein P, Zimmermann R, Haslinger C. Retained placenta and postpartum hemorrhage: Time is not everything. Arch Gynecol Obstet 2021;304:903-11.
[Crossref] [Google Scholar] [PubMed]
- Findlay RJ, Macrae EH, Whyte IY, Easton C, Forrest LJ. How the menstrual cycle and menstruation affect sporting performance: Experiences and perceptions of elite female rugby players. Br J Sports Med 2020;54(18):1108-13.
[Crossref] [Google Scholar] [PubMed]
- Harmsen MJ, Wong CF, Mijatovic V, Griffioen AW, Groenman F, Hehenkamp, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: A systematic review. Hum Reprod Update 2019;25(5):647-71.
[Crossref] [Google Scholar] [PubMed]
- Carugno J. Clinical management of vaginal bleeding in postmenopausal women. Climacteric 2020;23(4):343-9.
[Crossref] [Google Scholar] [PubMed]
- Munro MG, Mast AE, Powers JM, Kouides PA, O’Brien SH, Richards T, et al . The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol 2023;229(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Pacheco LD, Clifton RG, Saade GR, Weiner SJ, Parry S, Thorp Jr JM, et al. Tranexamic acid to prevent obstetrical hemorrhage after cesarean delivery. N Engl J Med 2023;388(15):1365-75.
[Crossref] [Google Scholar] [PubMed]
- Osuga Y, Enya K, Kudou K, Tanimoto M, Hoshiai H. Oral gonadotropin-releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: A randomized controlled trial. Obstet Gynecol 2019;133(3):423-33.
[Crossref] [Google Scholar] [PubMed]
- Ramalho I, Leite H, Aguas F. Abnormal uterine bleeding in adolescents: A multidisciplinary approach. Acta Med Port 2021;34(4):291-7.
[Crossref] [Google Scholar] [PubMed]
- Barra F, Vitale SG, Seca M, Scala C, Leone RMU, Cianci A, et al. The potential role of elagolix for treating uterine bleeding associated to uterine myomas. Expert Opin Pharmacother 2020;21(12):1419-30.
[Crossref] [Google Scholar] [PubMed]
- McLintock C. Prevention and treatment of postpartum hemorrhage: Focus on hematological aspects of management. Hematol Am Soc Hematol Educ Progr 2020;2020(1):542-6.
[Crossref] [Google Scholar] [PubMed]
- Gynecotokology. World core medical journal abstract (Journal of Obstetrics and Gynecology), 2006;2(10):51-62.
- Zhang Yingzi. Comparison of the clinical efficacy and safety of different doses of progesterone when treating functional uterine bleeding. J Clin Rational Drug Use 2021;14(11):117-8.
- Chodankar R, Critchley HO. Biomarkers in abnormal uterine bleeding. Biol Reprod 2019;101(6):1155-66.
[Crossref] [Google Scholar] [PubMed]
- Vijayaraghavan Sr A, Jadhav C, Pradeep B, Bindu H, Kumaran S, Pradeep BK, et al. A histopathological study of endometrial biopsy samples in abnormal uterine bleeding. Cureus 2022;14(11).
[Crossref] [Google Scholar] [PubMed]
- Sahu HD, Varma AV, Karmarkar S, Malukani K, Khanuja A, Kesharwani P, et al. Endometrial histopathology in abnormal uterine bleeding and its relation with thyroid profile and endometrial thickness. Cureus 2023;15(4).
[Crossref] [Google Scholar] [PubMed]
- Harroche A, Meunier S, Falaise C, Da Costa S, Oudot C. Heavy menstrual bleeding in teenage girls and women with inherited bleeding disorders. Rev Prat 2019;69(4):417-22.
[Google Scholar] [PubMed]
- Rytkonen KT. Glycolysis and heavy menstrual bleeding. Reprod Sci 2023;30(6):2016-8.
- Marnach ML, Laughlin-Tommaso SK. Evaluation and management of abnormal uterine bleeding. Mayo Clin Proc 2019;94(2):326-35.
[Crossref] [Google Scholar] [PubMed]
- Iweha C, Graham A, Cui W, Marsh C, Nothnick WB. The uterine natural killer cell, cytotoxic T lymphocyte, and granulysin levels are elevated in the endometrium of women with nonstructural abnormal uterine bleeding. FS Sci 2022;3(3):246-54.
[Crossref] [Google Scholar] [PubMed]
- Kimura F, Takahashi A, Kitazawa J, Yoshino F, Katsura D, Amano T, et al. Successful conservative treatment for massive uterine bleeding with non-septic disseminated intravascular coagulation after termination of early pregnancy in a woman with huge adenomyosis: Case report. BMC Women's Health 2020;20:1-6.
[Crossref] [Google Scholar] [PubMed]
- Joshi BR, Rizal S, Subedi S. Thyroid dysfunction in patient with abnormal uterine bleeding in a tertiary hospital of eastern Nepal: A descriptive cross-sectional study. J Nepal Med Assoc 2021;59(239):635.
[Crossref] [Google Scholar] [PubMed]
- Murji A, Sanders AP, Monteiro I, Haiderbhai S, Matelski J, Walsh C, et al. Cesarean scar defects and abnormal uterine bleeding: A systematic review and meta-analysis. Fertil Steril 2022;118(4):758-66.
[Crossref] [Google Scholar] [PubMed]
- Akram Z, Mahjabeen I, Irshad F, Ahmed MW, Rehman S, Rizwan M, et al. Expression deregulation of matrix metalloproteinases and vasoconstriction related genes in Pakistani females with abnormal uterine bleeding. BMC Women's Health 2022;22(1):543.
- Mir SA, Ara R, Amin F, Malik A, Hamid L, Ali T, et al. Evaluation of the safety and efficacy of ormeloxifene, a selective estrogen receptor modulator and medroxyprogesterone acetate in women with non-structural abnormal uterine bleeding: A randomized clinical trial. Medicina 2022;58(11):1503.
[Crossref] [Google Scholar] [PubMed]
- Schlaff WD, Ackerman RT, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med 2020;382(4):328-40.
[Crossref] [Google Scholar] [PubMed]
- Bumbuliene Z, Sragyte D, Klimasenko J, Bumbul-Mazurek E. Abnormal uterine bleeding in adolescents: Ultrasound evaluation of uterine volume. Gynecol Endocrinol 2019;35(4):356-9.
[Crossref] [Google Scholar] [PubMed]