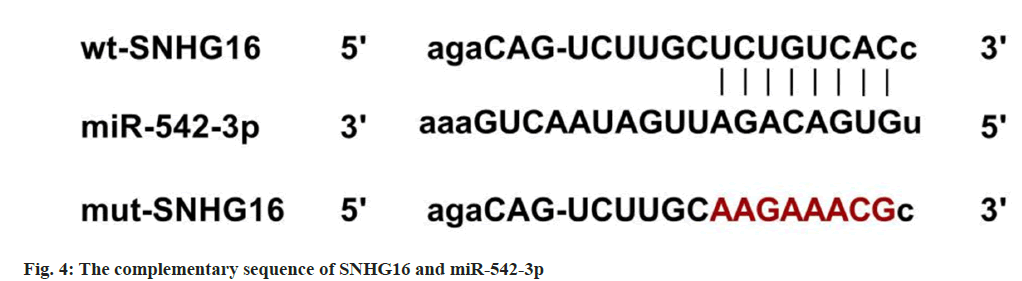- *Corresponding Author:
- Yan Liu
Department of Respiratory and Critical Care Medicine, Jiading District Central Hospital Affiliated Shanghai University of Medicine and Health Sciences, Jiading, Shanghai 201805, China
E-mail: liuyan19821127@163.com
| Date of Received | 19 January 2023 |
| Date of Revision | 07 August 2023 |
| Date of Acceptance | 22 January 2024 |
| Indian J Pharm Sci 2024;86(1):314-321 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of Senecio scandens ethanol extract on Streptococcus pneumoniae-evoked alveolar epithelial cell damage and its molecular mechanism. Alveolar epithelial cells and Streptococcus pneumonia infected alveolar epithelial cells were incubated with Senecio scandens ethanol extract. Western blotting measured cleaved caspase-3 protein expression. Quantitative reverse transcription-polymerase chain reaction detected SNHG16 and microRNA-542-3p levels, and the dual-luciferase reporter assay investigated the targeting relationship between them. After treatment with different concentrations of Senecio scandens ethanol extract, alveolar epithelial cell survival rate did not change, while the survival rate of alveolar epithelial cells infected by Streptococcus pneumoniae was gradually decreased, and the decrease was stable at 32 μg/ml. After treatment with 8, 16, 32 μg/ml Senecio scandens ethanol extract, the apoptotic rate and inflammation of alveolar epithelial cells infected by Streptococcus pneumoniae, inflammation was repressed. Senecio scandens ethanol extract dose-dependently reduced SNHG16, but elevated microRNA-542-3p in cells. After SNHG16 knockdown, microRNA-542-3p levels were boosted, apoptotic rate was declined, and inflammation was inhibited (p<0.05). Overexpression of SNHG16 could reverse the effects of Senecio scandens ethanol extract on the apoptosis and inflammatory factors of alveolar epithelial cells infected by Streptococcus pneumoniae. SNHG16 targeted microRNA-542-3p. Senecio scandens ethanol extract could impair Streptococcus pneumoniae-evoked alveolar epithelial cell injuries by regulating the SNHG16/microRNA-542-3p axis.
Keywords
Senecio scandens ethanol extract, SNHG16, microRNA-542-3p, Streptococcus pneumoniae, alveolar epithelial cells, inflammation
Community Acquired Pneumonia (CAP) has a high mortality rate, with Streptococcus pneumoniae (S. pneumoniae) being its main pathogen. The main pathological changes of pneumonia caused by S. pneumoniae infection are apoptosis and inflammatory response in Alveolar Epithelial Cells (AECs)[1,2]. Research has found that Traditional Chinese Medicine (TCM) is benefit to CAP treatment[3,4]. Senecio scandens (S. scandens) (also known as Qianli Gunag) is a perennial climbing herb belonging to the family Compositae, and has excellent clinical therapeutic effects in various diseases. For instance, water extract of S. scandens could suppress Mas-Related G Protein- Coupled Receptor-B2 (MRGPRB2) receptor of mast cells to alleviate pruritus[5]. S. scandens has known antibacterial properties, and exhibits suppression on S. pneumoniae[6]. In addition, the ethanol extract of S. scandens could elevate MUC2 and sIgA, and maintain the diversity of gut microbiota[6]. However, the effects and mechanisms of S. scandens ethanol extract on the apoptosis and inflammatory response of S. pneumoniae infected AECs are still unclear.
SNHG16 is a functional long noncoding Ribonuclic Acid (lncRNA), and was discovered to promote Lipopolysaccharide (LPS)-evoked inflammation and apoptosis in acute pneumonia[7]. SNHG16 could abolish microRNA (miR)-15a/16 cluster-induced down-regulation of inflammatory pathway[8]. SNHG16 promoted inflammatory reaction and macrophage proliferation in atherosclerosis patients[9]. Thus, these data suggested that SNHG16 was involved in regulating acute pneumonia and inflammatory response. However, whether S. scandens ethanol extract regulated CAP via SNHG16 remain unclear. Here, this work used S. pneumoniae infected AECs to mimic CAP in vitro, and investigated the action and relationship of S. scandens ethanol extract and SNHG16 in cell apoptosis and inflammation, and then explored their underlying mechanism.
Materials and Methods
Experimental materials:
Pulmonary AECs (Hepapic), S. pneumoniae (American Type Culture Collection (ATCC), United States of America (USA)); Roswell Park Memorial Institute (RPMI)-1640 medium and Lipofectamine 3000 (Sigma, USA); S. scandens (Bozhou Pharmaceutical Market, China); SNHG16 siRNAs (si-SNHG16), overexpressing plasmids (plasmid cloning Deoxyribonucleic Acid (pcDNA)- SNHG16), miR-542-3p mimic, si-NC, miR-NC (RiboBio, China); 3-(4,5-Dimethylthiazol-2- yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) kit and apoptosis detection assay kit (Shanghai Jingke Chemical Technology Co., Ltd.); protein extraction kit (Shanghai Beibo Biotechnology Co., Ltd.); cleaved caspase-3 (ab2302) and secondary antibodies (Abcam, USA); Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL)- 6 and IL-10 Enzyme-Linked Immunosorbent assay (ELISA) kits (Nanjing Saihongrui Biotechnology Co., Ltd.); Trizol and quantitative Reverse Transcription Polymerase Chain Reaction (qRTPCR) kit (Shanghai Caiyou Industrial Co., Ltd.); dual-luciferase reporter assay kit (Wuhan Purity Biotechnology Co., Ltd.).
Preparation of ethanol extract from S. scandens:
An appropriate amount of dried whole grass of S. scandens was took and crushed, the impurities were removed through a 20 mesh sieve. About 200 g samples were incubated with 2 l of 70 % ethanol for 1 h, and soaked using an ultrasonic instrument to shake for 1 h. After heating in a microwave for 3 min, the mixture was soaked for additional 24 h. The residue was filtered, and the leachate was collected and added with 50 ml of anhydrous ethanol to the filtrate and filter again. Then the ethanol was recovered by reducing pressure, and the ethanol extract was obtained. The extraction was added with 1/3rd volume of ether, and refrigerated at 4° for 6 h, the extracts were took out and filtered again. Finally, the extracts were placed into -46° refrigerator for 12 h, and a dry powder was collected, and 1 g/ml of raw medicine was prepared for later use.
Cell culture, treatment and transfection:
Hepapic cells were maintained in 5 % Carbon dioxide (CO2) at 37° with RPMI-1640 medium. Hepapic cells were infected with 1×108 CFU/ ml S. pneumoniae to mimic CAP in vitro (model group), with the untreated cells as control group. For transfection, Hepapic cells were transfected with oligonucleotides and/or plasmids based on the experimental design, followed by indicated treatment.
MTT assay:
Hepapic cells infected with S. pneumoniae or not were incubated with 0, 1, 2, 4, 8, 16, 32, 64, or 128 μg/ml ethanol extract, respectively. 48 h later, cells at 3×104 per well were inoculated on a 96 well plate overnight, followed by reacting with 20 μl MTT (5 g/l) for 4 h. Where after, each well was incubated with 150 μl Dimethyl Sulfoxide (DMSO) for 2 h lastly, the absorbance at 490 nm was examined.
Flow cytometry:
Hepapic cells were consecutively reacted with 5 μl Annexin V-Fluorescein Isothiocyanate (FITC) and 5 μl Propidium Iodide (PI) for 10 min avoiding light, followed by detecting cell apoptosis using the flow cytometry.
Western blotting:
Total extracted proteins were separated and underwent membrane transfer. Then cleaved caspase-3 (ab2302, 1:2000) was added and incubated with membranes at 4° for 12 h, and secondary antibody was used to incubate with members for 2 h. The gray values of the protein bands were analyzed.
ELISA:
After indicating treatment, Hepapic cell supernatant was taken from each group, and the Optical Density (OD) 450 nm value of each group was detected by enzyme label.
qRT-PCR:
The total RNAs of Hepapic cells were extracted using Trizol, and the NanoDrop 2000c spectrophotometer was applied for detecting the purity of RNAs. Then reverse transcription was conducted to generate complementary DNA (cDNA), which were then used for qRT-PCR reaction following the conditions; 95° 2 min, 95° denaturation 15 s, 60° 30 s, 72° 30 s, a total of 40 cycles. The fold changes were assessed using 2-ΔΔCt method. The primers for qRT-PCR: SNHG16, forward 5'-CAGAATGCCATGGTTTCCCC-3'; reverse 5'-TGGCAAGAGACTTCCTGAGG-3', Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH); forward 5 ' - C C A C AT C G C T C A G A C A C C AT- 3 ' ; reverse 5'-ACCAGGCGCCCAATACG-3'; miR-542-3p forward 5'-TGTGACAGATTGATAACTGAAA-3'; reverse 5'-GTGCAGGGTCCGAGGT-3’; U6 forward 5'-CGCTTCGGCAGCACATATACTA-3’ and reverse 5'-CGCTTCACGAATTTGCGTGTCA-3'.
Dual-luciferase reporter assay:
The predicted complementary sequences between SNHG16 and miR-542-3p and the corresponding mutant sequence were amplified to establish Wild Type (WT)-SNHG16 and mut-SNHG16 luciferase reporter vector. All plasmids were co-transfected with miR-132-3p or miR-NC into Hepapic cells. 48 h later, the detection of the relative luciferase was conducted.
Statistical analysis:
The data were manifested by x̄ ±s. The Analysis of Variance (ANOVA) or t-test was applied for the comparison in groups. p<0.05 meant significant difference.
Results and Discussion
As exhibited in fig. 1, the survival rate of Hepapic cells after treatment with different concentrations of S. scandens ethanol extract had no change; while Hepapic cells survival rate were decreased gradually by S. pneumoniae infection (model group), and the decrease was stabilized when the concentration of S. scandens ethanol extract was >32 μg/ml.
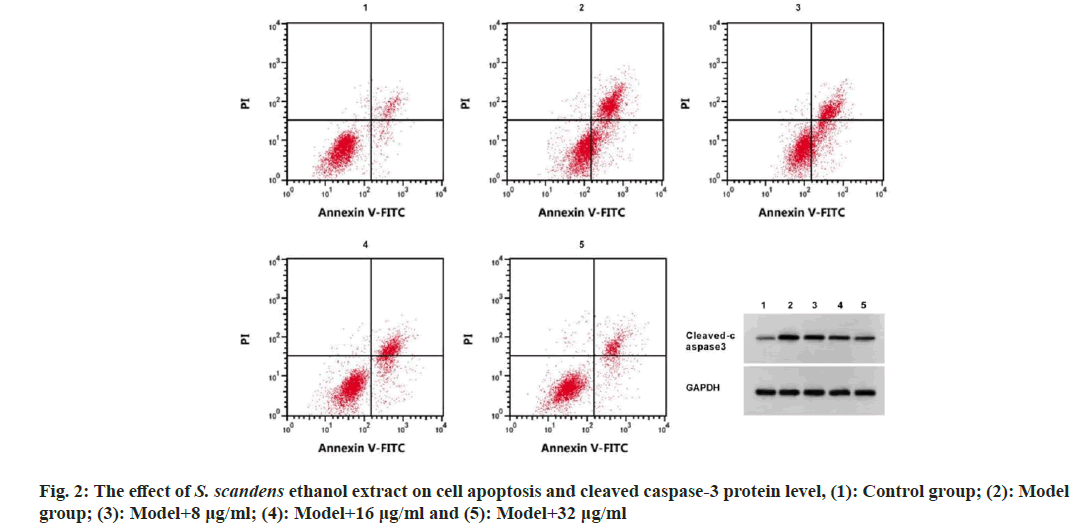
The apoptosis rate, cleaved caspase-3 protein levels and TNF-α and IL-6 levels were rose, while IL-10 levels were declined in the model group (p<0.05). Then cells in model group was treated with 8, 16, and 32 μg/ml S. scandens ethanol extract, it was found cell apoptosis rate, cleaved caspase-3 protein levels, as well as TNF-α and IL-6 levels were reduced, and IL-10 levels were boosted in cells in a concentration dependent manner (p<0.05) (fig. 2 and Table 1).
| Group | Apoptosis rate (%) | Cleaved caspase-3 | pg/ml | ||
|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-10 | |||
| Control | 6.65±0.23 | 0.10±0.01 | 64.37±5.57 | 122.57±10.74 | 359.14±25.78 |
| Model | 26.40±0.921 | 0.79±0.061 | 302.21±17.131 | 541.81±25.771 | 46.20±5.641 |
| Model+8 μg/ml | 21.65±1.062 | 0.60±0.052 | 258.12±14.062 | 480.19±17.402 | 81.15±3.922 |
| Model+16 μg/ml | 17.95±0.672,3 | 0.42±0.032,3 | 177.50±10.922,3 | 356.75±12.642,3 | 170.65±12.712,3 |
| Model+32 μg/ml | 13.94±0.352,3,4 | 0.25±0.032,3,4 | 115.78±5.922,3,4 | 230.76±12.052,3,4 | 285.37±10.002,3,4 |
| F | 375.907 | 140.569 | 213.291 | 323.494 | 271.683 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: 1p<0.05 vs. control group; 2p<0.05 vs. model group; 3p<0.05 vs. model group+8 μg/ml and 4p<0.05 vs. model group+16 μg/ml
Table 1: Detection of Apoptosis and Inflammatory Factors by S. Scandens Ethanol Extract (X̄±S, N=3)
The levels of SNHG16 were boosted and miR- 542-3p contents were declined in the model group (p<0.05). After S. scandens ethanol extract treatment, SNHG16 level was decreased, and miR-542-3p level was elevated in the model+8, 16 and 32 μg/ml groups in a dose-dependent manner (p<0.05) (Table 2).
| Group | SNHG16 | miR-542-3p |
|---|---|---|
| Control | 1.00±0.00 | 1.00±0.00 |
| Model | 4.29±0.071 | 0.12±0.011 |
| Model+8 μg/ml | 3.83±0.062 | 0.24±0.022 |
| Model+16 μg/ml | 3.09±0.032,3 | 0.45±0.032,3 |
| Model+32 μg/ml | 1.76±0.052,3,4 | 0.75±0.052,3,4 |
| F | 2422.221 | 506.037 |
| p | 0.000 | 0.000 |
Note: 1p<0.05 vs. control group; 2p<0.05 vs. model group; 3p<0.05 vs. model group+8 μg/ml and 4p<0.05 vs. model group+16 μg/ml
Table 2: Measurement of Snhg16 and Mir-542-3p Content (X̄±S, N=3)
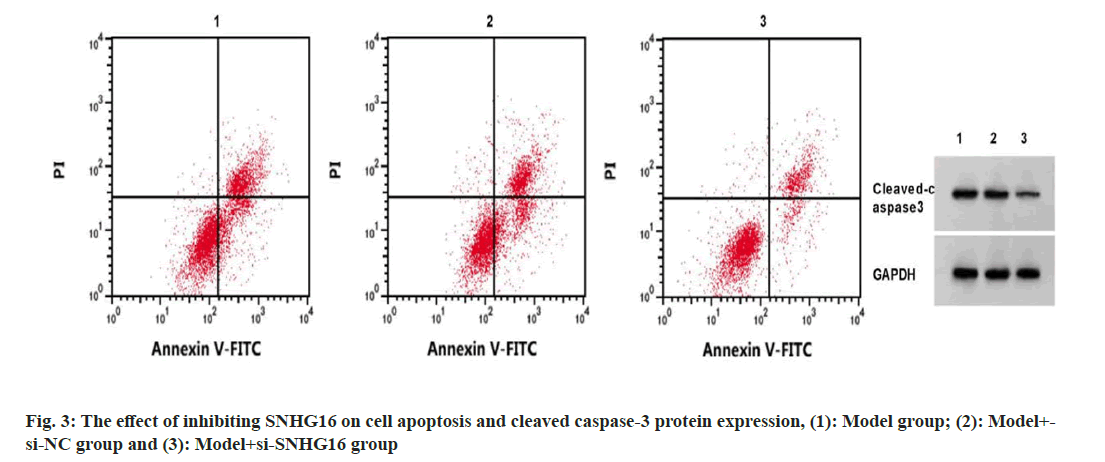
SNHG16 expression in the model+si-SNHG16 group was decreased, while miR-542-3p content was increased, the apoptosis rate and cleaved caspase-3 protein levels were reduced, and the contents of IL-6 and TNF-α were decreased, and IL-10 content was increased (p<0.05) (fig. 3 and Table 3).
| Group | SNHG16 | miR-542-3p | Apoptosis rate (%) | Cleaved caspase-3 | pg/ml | ||
|---|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-10 | |||||
| Model | 1.00±0.00 | 1.00±0.00 | 26.50±0.91 | 0.79±0.07 | 306.65±16.92 | 545.98±31.31 | 46.13±6.47 |
| Model+si-NC | 0.97±0.03 | 1.01±0.02 | 26.47±0.93 | 0.79±0.05 | 309.61±20.85 | 545.39±33.64 | 46.02±6.30 |
| Model+si-SNHG16 | 0.24±0.021,2 | 7.28±0.221,2 | 9.21±0.331,2 | 0.15±0.011,2 | 84.82±5.681,2 | 167.07±10.211,2 | 319.96±17.571,2 |
| F | 1282.375 | 2420.637 | 496.853 | 163.840 | 198.620 | 194.047 | 576.645 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: 1p<0.05 vs. model group and 2p<0.05 vs. model+si-NC
Table 3: Inhibition of Snhg16 on the Detection of Cell Apoptosis and Inflammatory Factors (X̄±S, N=3)
Star base predicted the complementary sequences of miR-542-3p on SNHG16 (fig. 4). The luciferase activity was notably declined in cells transfected with WT-SNHG16 and miR-542-3p (p<0.05) (Table 4).
| Group | WT-SNHG16 | MUT-SNHG16 |
|---|---|---|
| miR-NC | 1.03±0.07 | 1.01±0.08 |
| miR-542-3p | 0.24±0.021 | 0.98±0.05 |
| t | 18.795 | 0.551 |
| p | 0.000 | 0.611 |
Note: 1p<0.05 vs. miR-NC group
Table 4: Dual-Luciferase Reporter Assay
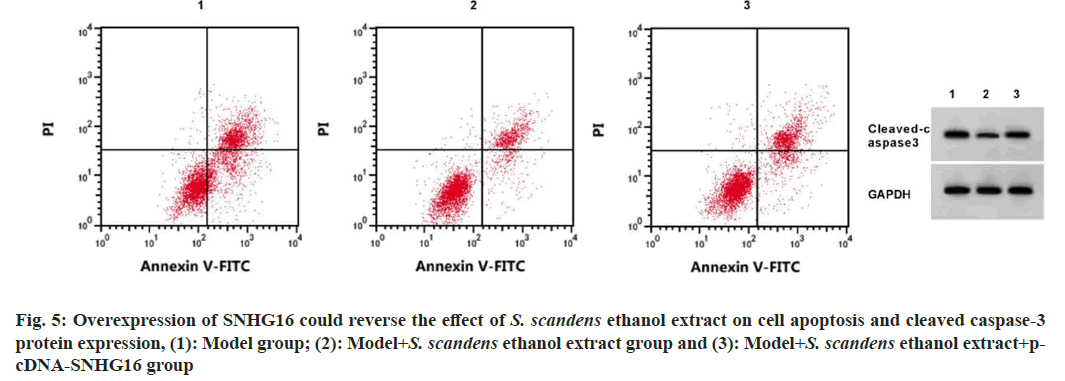
SNHG16 expression level was decreased, miR- 542-3p content was increased, apoptosis rate and cleaved caspase-3 protein expression levels were decreased, IL-6 and TNF-α levels were descended, and IL-10 content was boosted in model+ethanol extract (p<0.05). SNHG16 content was increased, miR-542-3p level was declined, the apoptosis rate, cleaved caspase-3 protein levels were increased, and IL-6 and TNF-α levels were boosted, while IL-10 content were descended (p<0.05) in the model+ethanol extract+pcDNA-SNHG16 group relative to the model+ethanol extract group, (fig. 5 and Table 5).
| Group | SNHG16 | miR-542-3p | Apoptosis rate (%) | Cleaved caspase-3 | pg/ml | ||
|---|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-10 | |||||
| Model | 4.33±0.07 | 1.00±0.00 | 26.58±0.92 | 0.78±0.05 | 306.15±18.08 | 546.44±30.43 | 47.58±5.68 |
| Model+ethanol extract | 1.77±0.081 | 6.26±0.081 | 13.89±0.331 | 0.24±0.021 | 114.85±6.751 | 239.24±14.051 | 286.29±9.061 |
| Model+ethanol extract+pcDNA-SNHG16 | 4.10±0.102 | 1.48±0.122 | 23.73±0.682 | 0.67±0.032 | 282.44±13.272 | 524.83±9.052 | 65.94±5.442 |
| F | 847.563 | 3659.596 | 281.425 | 192.868 | 178.412 | 219.533 | 1103.314 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: 1p<0.05 vs. model group and 2p<0.05 vs. model+ethanol extract group
Table 5: Overexpression of Snhg16 Could Reverse the Effects of S. Scandens Ethanol Extract on Cell Apoptosis and Inflammatory Factors
Currently, TCM treatment has great advantages and characteristics, providing new ideas for clinical treatment in CAP. As a common TCM, S. scandens and its extracts and compounds possess diverse pharmaceutical activities, like anti-inflammatory, antibacterial and anticancer activities[6,10]. The petroleum ether extract of S. scandens could impede inflammatory response in LPS-stimulated RAW264.7 cells[11]. In our work, we used S. pneumoniae-stimulated Hepapic cells to mimic pneumonia condition in vitro, followed by treating with different doses of ethanol extracts of S. scandens. We discovered that there was no significant change in the survival rate of Hepapic cells after S. scandens treatment, while Hepapic cell survival rates were decreased by S. pneumoniae stimulation, but the decrease was suppressed when the concentration of S. scandens was greater than 32 μg/ml, implying that S. scandens ethanol extract could affect S. pneumoniae infected AECs at a concentration of 32 μg/ml, therefore, 8, 16, and 32 μg/ml ethanol extract were selected for subsequent analyses. Functionally, we proved that S. scandens ethanol extract suppressed S. pneumoniae induced apoptosis and inflammation in AECs
In recent years, with the increasingly widespread bacterial resistance, the difficulty of treating infectious diseases has increased dramatically. To address this issue, researchers have attempted a combination of TCM and Western medicine antimicrobial therapy. TCM can regulate the biofilm of drug-resistant bacteria by inhibiting quorum sensing system expression, bacterial adhesion, and other aspects. It does not directly kill bacteria, but inhibits communication between bacteria and reduces their infectivity. However, the antibacterial effect of TCM alone is limited, so in clinical applications, TCM is often applied in combination with antibiotics. An increasing number of studies have shown that the combination of TCM and Western medicine can better inhibit the quorum sensing system of drug-resistant bacteria, thereby reducing biofilm formation and improving the antibacterial effect of antibiotics. Thereafter, we also found that S. scandens ethanol extract led to a decrease of SNHG16 expression in model cells. SNHG16 deficiency impaired LPS-evoked apoptotic and inflammatory injuries by modulating miR-370-3p[12]. SNHG16 deletion partially alleviated LPS triggered autophagy, apoptosis, and inflammation[13]. These data suggested the potential involvement of SNHG16 in regulating alveolar epithelial cell function infected with S. pneumoniae. In this work, we proved that SNHG16 silencing could suppress the apoptosis and inflammatory reaction. Moreover, forced expression of SNHG16 could abolish the suppressing action of S. scandens ethanol extract on the apoptosis and inflammation evoked by S. pneumoniae. In addition, through bioinformatics analysis, we proved that SNHG16 bound to miR- 542-3p. miR-542-3p is an important biomarker for CAP and pneumonia-associated sepsis[14]. The extracellular vesicle miR-542-3p impaired the inflammation in ischemic glial cells[15]. miR- 542-3p functioned in regulating antiviral and inflammatory responses[16]. Buxin Ruanmai granules could repress the inflammatory reaction and oxidative stress of myocardial cells through the miR-542-3p/GABARAP axis[17]. All the results indicated the implication of miR-542-3p in CAP. Here, we also discovered that ethanol extract of S. scandens increased miR-542-3p expression in model cells. However, whether miR-542-3p was involved in the modulatory action of SNHG16 and S. scandens ethanol extract in S. pneumoniae -treated AECs needs further study.
In conclusion, S. scandens ethanol extract could suppress S. pneumoniae evoked apoptotic and inflammatory injuries in AECs, suggesting the potential application of S. scandens ethanol extract in pneumonia treatment.
Authors’ contribution:
Yan Liu and Zhengya Wang have contributed equally to this study
Conflict of interests:
The authors declared no conflict of interests.
References
- Goekeri C, Pennitz P, Groenewald W, Behrendt U, Kirsten H, Zobel CM, et al. microRNA-223 dampens pulmonary inflammation during pneumococcal pneumonia. Cells 2023;12(6):959.
[Crossref] [Google Scholar] [PubMed]
- Qinghe G, Guangyu L, Xiaobin L, Qingsong H. Baicalein exerts a protective role in pneumonia caused by Streptococcus pneumoniae. Front Biosci 2019;24(5):849-58.
[Crossref] [Google Scholar] [PubMed]
- Wang M, Liu H, Chen Y, Yu J, Lin J, Sun Z, et al. Guideline on treating community-acquired pneumonia with Chinese patent medicines. Pharmacol Res 2023;196:106919.
[Crossref] [Google Scholar] [PubMed]
- An L, Lin L, Wang S, Xie T, Yang Y, Zhai W, et al. Plasma characteristic metabolites of pediatric community-acquired pneumonia in traditional Chinese medicine syndrome differentiation. Anat Rec 2021;304(11):2579-91.
[Crossref] [Google Scholar] [PubMed]
- Ye F, Jiang Y, Zhang J, Zong Y, Yu M, Chen C, et al. Water extract of Senecio scandens Buch.-Ham ameliorates pruritus by inhibiting MrgprB2 receptor. J Inflamm Res 2022;15:5989-98.
[Crossref] [Google Scholar] [PubMed]
- Ngai HL, Yang X, Chu AJ, Harper R, Jacobsen AB, Lau DT, et al. Multi-methodological approach for the quality assessment of Senecionis scandentis Herba (Qianliguang) in the herbal market. Plos One 2022;17(4):e0267143.
[Crossref] [Google Scholar] [PubMed]
- Zhou Z, Zhu Y, Gao G, Zhang Y. Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS-induced WI-38 cell apoptosis and inflammation in acute pneumonia. Life Sci 2019;228:189-97.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Lou C, Gao J, Zhang X, Du Y. LncRNA SNHG16 reverses the effects of miR-15a/16 on LPS-induced inflammatory pathway. Biomed Pharmacother 2018;106:1661-7.
[Crossref] [Google Scholar] [PubMed]
- An JH, Chen ZY, Ma QL, Wang HJ, Zhang JQ, Shi FW. LncRNA SNHG16 promoted proliferation and inflammatory response of macrophages through miR-17-5p/NF-?B signaling pathway in patients with atherosclerosis. Eur Rev Med Pharmacol Sci 2019;23(19):8665-77.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Huang L, Chen S. Senecio scandens Buch.-Ham: A review on its ethnopharmacology, phytochemistry, pharmacology, and toxicity. J Ethnopharmacol 2013;149(1):1-23.
[Crossref] [Google Scholar] [PubMed]
- Yang HH, Shen JH, Liu QH. Inhibitory effect of petroleum ether extract of Qianli Guang on LPS activated inflammatory response. J Huazhong Agric Univ 2020;9(6):193-7.
- Zhang J, Mao F, Zhao G, Wang H, Yan X, Zhang Q. Long non-coding RNA SNHG16 promotes lipopolysaccharides-induced acute pneumonia in A549 cells via targeting miR-370-3p/IGF2 axis. Int Immunopharmacol 2020;78:106065.
[Crossref] [Google Scholar] [PubMed]
- Xia L, Zhu G, Huang H, He Y, Liu X. LncRNA small nucleolar RNA host gene 16 (SNHG16) silencing protects lipopolysaccharide (LPS)-induced cell injury in human lung fibroblasts WI-38 through acting as miR-141-3p sponge. Biosci Biotechnol Biochem 2021;85(5):1077-87.
[Crossref] [Google Scholar] [PubMed]
- Hermann S, Brandes F, Kirchner B, Buschmann D, Borrmann M, Klein M, et al. Diagnostic potential of circulating cell-free microRNAs for community-acquired pneumonia and pneumonia-related sepsis. J Cell Mol Med 2020;24(20):12054-64.
[Crossref] [Google Scholar] [PubMed]
- Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S, et al. Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther 2021;12(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Herbert C, Sebesfi M, Zeng QX, Oliver BG, Foster PS, Kumar RK. Using multiple online databases to help identify micro RNA s regulating the airway epithelial cell response to a virus-like stimulus. Respirology 2015;20(8):1206-12.
[Crossref] [Google Scholar] [PubMed]
- Yan D, Zhao LL, Yue BW, Qian H, Zhang ZH, Wang N, et al. Granule of BU-XIN RUAN-MAI attenuates the patients’ angina pectoris of coronary heart disease via regulating miR-542-3p/GABARAP signaling. Evid Based Complement Altern Med 2019;2019:1808419.
[Crossref] [Google Scholar] [PubMed]
 ): Hepapic and (
): Hepapic and ( ): Streptococcus pneumoniae+Hepapic
): Streptococcus pneumoniae+Hepapic