- *Corresponding Author:
- M. S. Moqbel
Department of Anatomy, College of Veterinary Medicine, King Faisal University, Al-Ahsa 31982, Saudi Arabia
E-mail: m.s.moqbel@hotmail.com
| Date of Received | 19 January 2021 |
| Date of Revision | 06 January 2022 |
| Date of Acceptance | 05 September 2022 |
| Indian J Pharm Sci 2022;84(5):1233-1240 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study was conducted to determine the antidiabetic effect of Momordica charantia on pancreatic islets of Langerhans in streptozotocin-induced diabetic male Wistar rats in relation to the distribution of insulin immuno-positive beta cells. Momordica charantia was fed to rats at 2 %, 5 % and 10 % of the standard diet for 12 w. After sacrifice, the pancreatic tissues were obtained for histopathological and immunohistochemical observations. In addition, monitoring of fasting blood glucose was applied each month during experimental period. The results revealed that the oral doses of Momordica charantia at 5 % and 10 % of the daily diet increased the percentage of insulin-positive pancreatic beta cells as well as the size and number of pancreatic islets. Moreover, significant (p≤0.01) dose and time-dependent reduction in fasting blood glucose levels were observed. In conclusion, these results suggest that Momordica charantia induces antidiabetic effects via regeneration of insulin-positive pancreatic beta cells and increase the number of insulin secretory granules; hence, blood glucose reduction.
Keywords
Antidiabetic, glucose, immunohistochemistry, insulin, Momordica charantia, pancreatic beta cells
Chronic metabolic disorders such as Diabetes Mellitus (DM) is considered a critical clinical disease in the world. It has been estimated that 451 million people had diabetes in 2017 among populations and is expected to rise to 693 million people worldwide in 2045[1]. Hyperglycemia is a common metabolic symptom of DM, caused by a relative or absolute lack of insulin secretion or insulin resistance[2]. DM is classified according to insulin secretion into Type 1 DM (T1DM, absolute lack) and Type 2 DM (T2DM, insufficient secretion). In this respect, T2MD represents 90 % of diabetic cases[3]. The Kingdom of Saudi Arabia (KSA) has a high prevalence of DM among people that caused a major local health problem. In 2011, KSA was classified as the top 10 globally in the highest prevalence of diabetic countries[4].
Insulin is a hormone produced from pancreatic beta cells and binds with tissue insulin receptor to control blood glucose level by enhancement tissue glucose utilizing and glycogen synthesis as well as reduction of hepatic glycogenolysis and gluconeogenesis[5]. Therefore, total loss or reduced numbers of pancreatic beta cells lead to insulin reduction and subsequently diabetes exhibition[2]. DM treatment drugs included insulin and anti-hyperglycemic drugs (sulphonylurea derivatives, biguanides and thiazolidinediones) have been reported to rise several side effects[6]. Currently, several medicinal plants have been documented to possess promised treated effects against T2DM or its symptoms without known side effects[7-9].
Momordica charantia (MC) known as bitter melon, one of those medicinal plants, has been used as traditional medicine to control hyperglycemia. Several studies have established the antidiabetic effect of MC in Streptozotocin (STZ) and alloxan-induced T2DM in different animal models[10-12] as well as clinical trials in patients[7,13,14] . Previously, our lab reported that oral feeding of MC resulted in improvement in pancreatic, hepatic and renal functions by alleviation of STZ induced diabetic lesions[11]. In addition, MC has been proved to have potential effect as an antioxidant, antiinflammatory, anti-cancer, anti-obesity, antiulcer and anti-osteoporosis[15-19].
Numerous active ingredients of MC such as triterpenoids, polysaccharides, alkaloids, insulin‐like peptide, charantin, saponins, flavonoids and other polypeptides have been investigated and documented to have hypoglycemic effects[10,20-22]. Moreover, different suggested antidiabetic mechanisms of MC such as increasing insulin secretion, decreasing insulin resistance, and increasing peripheral and skeletal muscle cell glucose utilization have been investigated[20,23-26]. However, more researches are required to investigate the antidiabetic mechanism of MC on pancreatic beta cells. Thus, the objective of this study is to investigate the effect of MC on pancreatic islets emphasizes with insulin immuno-positive beta cells in STZ induced diabetic rats.
Materials and Methods
Plant:
Fresh whole fruits of MC were procured from local vegetable markets in Al-Ahsa, Eastern Province, KSA. The fruits were sliced, oven-dried at 60° temperature for 24 h, powdered and then added to the powdered feed at 2 %, 5 % and 10%. The mixture was then made as pellets[11].
Chemicals and materials:
STZ was purchased from Sigma Chemical Co, S0130, USA and a OneTouch® ultra-blood glucose meter was procured from Lifescan, Milpitas, California (CA), United States of America (USA).
Animals:
Fifty male Wistar albino rats of specific pathogen free grade weighing (150-200) g were used in this study. Rats were obtained from Laboratory Animals Center, College of Veterinary Medicine, King Faisal University, Al-Ahsa, KSA. Animals were housed in hygienic fiberglass cages, 10 rats per cage, with a 12 h light and dark cycle at 22°±2° and (55±5) % relative humidity and were fed on balanced commercial pellets obtained from the Grain Silos and Flourmills Organization, Riyadh, KSA. Before the experiment, all rats were acclimatized for 2 w in laboratory conditions with free access to food and water. All the experimental procedure and protocol was approved by the Animal Ethics Committee of Deanship of Scientific Research, King Faisal University, Al-Ahsa, KSA.
Induction of diabetic rats:
A freshly prepared single dose (85 mg/kg body weight) of STZ (Sigma Chemical Co, S0130, USA) was injected intra-peritoneal in all rats. After 72 h of STZ injection, fasting blood was collected from the tail vein for measuring the fasting blood glucose level. Rats were exhibited fasting blood glucose levels within 500 mg/dl, were considered as diabetic and were set subsequently in the experiment[11,27].
Experimental design:
The diabetic rats were randomly allotted into four groups of ten rats each group and normal rats (n=10) were used in the fifth group as a negative control. Negative control G1 was given untreated diet and water ad-libitum; while, the G2 was given STZ only and was kept as a positive control. G3, G4 and G5 were given STZ then daily fed with MC at 2 %, 5 % and 10 % of the standard diet respectively. During the experimental period (12 w), fasting blood glucose levels were measured once a month (4 w). At the end of the experiment, rats were humanly sacrificed and pancreatic tissue specimens were collected and fixed in 10 % buffered formalin for histopathological and immunohistochemical studies.
Measurement of fasting blood glucose levels:
Fasting blood was collected from the tail vein for measuring glucose levels each month (4 w) during the experimental period, using a OneTouch® ultra-blood glucose meter (Lifescan, Milpitas, CA, USA) according to the manufacturer instruction.
Histopathological technique:
For histopathological examination, conventional Hematoxylin and Eosin (H&E) stain was applied according to Bancroft[28]. Breifly, collected tissue were fixed in 10 % formalin for 48 h followed by immersion in 70 % alcohol for 72 h. Pancreatic tissue then trimmed and embedded in parafin wax using tissueprocessing machine (Leica, ASP300, Germany). After tissue sectioning (4 μm) on charged microscopic slides, the tissues were stained with H&E, dehydrated and coverslipped for microscopic examination.
Immunohistochemical technique:
Sectioned tissues were deparaffinised and rehydrated. Antigen retrieval was applied by microwaving in preheated sodium citrate buffer (pH 6) for 30 min. After retrieval, the sections were immersed in 1 % hydrogen peroxidase for 10 min were then covered with normal goat serum for 10 min after washing in Tris-Buffered Solution (TBS) 3 times for 5 min each. The primary antibody of insulin (Dako, Polyclonal Guinea Pig Anti- Insulin, A0564) was added for 18 h at 4°. After washing for in TBS, the secondary biotinylated antibody (Dako, rabbit anti-guinea pig immunoglobulins/Horseradish Peroxidase (HRP), P0141) was added for 10 min at room temperature. After washing, streptavidin- HRP conjugate (HSS-HRP) was added for 10 min and then incubated for 10 min with freshly prepared Diaminobenzidine (DAB) (chromogen solution). Counterstaining was applied for nuclei staining. Finally, the slides were dehydrated, cleared and mounted. The primary antibodies were omitted in negative control sections. All slides were examined using Olympus BX51, Japan for positive signals.
Statistical analysis:
Data were statistically evaluated by one way Analysis of Variance (ANOVA) and Duncan test using IBM Statistical Package for the Social Sciences (SPSS) V. 26 software. All results were expressed as mean±Standard Error (SE) and p<0.01 was identified as significantly different.
Results and Discussion
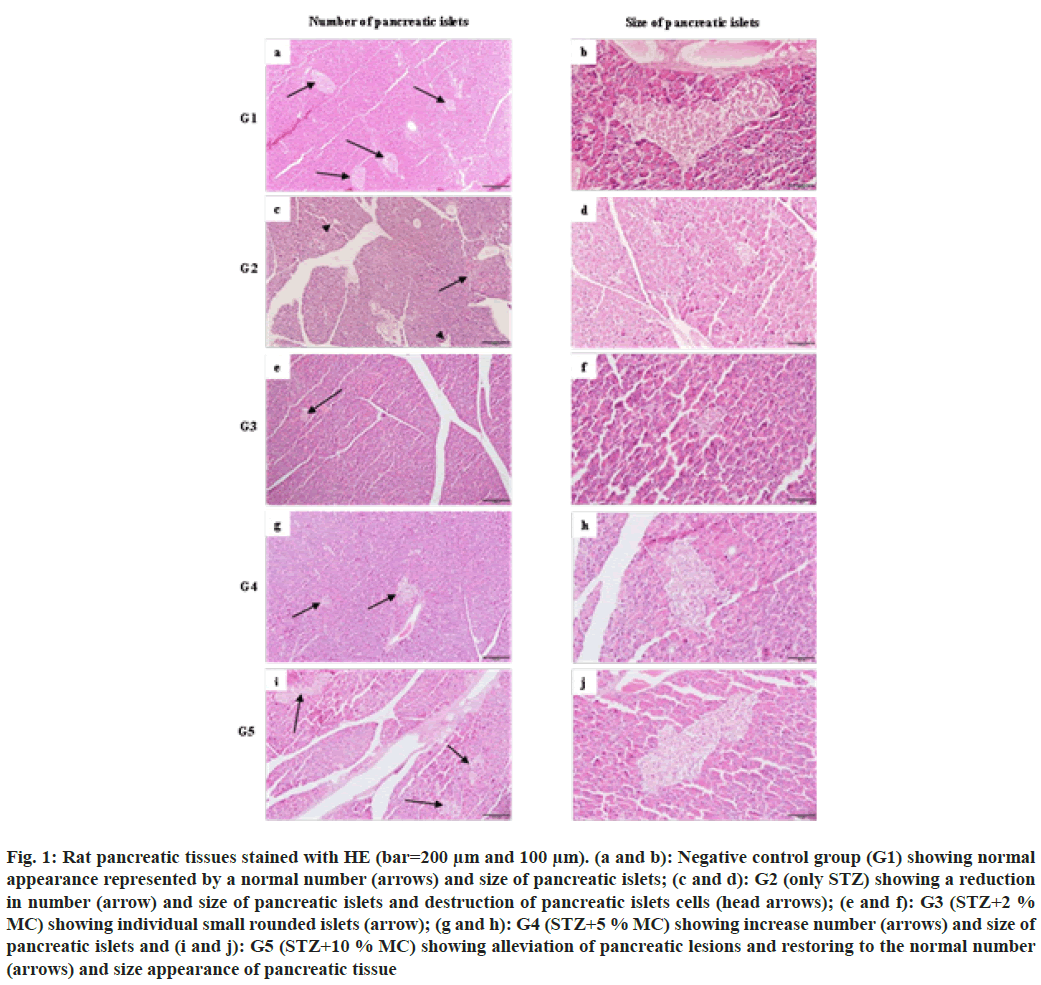
Tissues from the negative control group (G1) exhibited normal number, size and shape of pancreatic islets of Langerhans (fig. 1a and fig. 1b). However, the positive control group (G2) showed atrophy in the size and reduction in the number of pancreatic islets. In addition, destruction of pancreatic islets cells with few survival remaining cells (fig. 1c and fig. 1d). After 12 w of MC treatment, all treated groups showed dosedependent alleviation of pancreatic lesions caused by STZ induced diabetes. In group 3 (G3=STZ+2 % MC), mild restoring of pancreatic islets atrophy, represented by the appearance of individual small rounded islets, was observed (fig. 1e and fig. 1f). However, closely normal appearance of pancreatic islets, characterized by an increase in the number and size of pancreatic islets were seen in group 4 and group 5 (G4=STZ+5 % MC and G5=STZ+10 % MC respectively) (fig. 1g-fig. 1j).
Fig. 1: Rat pancreatic tissues stained with HE (bar=200 μm and 100 μm). (a and b): Negative control group (G1) showing normal appearance represented by a normal number (arrows) and size of pancreatic islets; (c and d): G2 (only STZ) showing a reduction in number (arrow) and size of pancreatic islets and destruction of pancreatic islets cells (head arrows); (e and f): G3 (STZ+2 % MC) showing individual small rounded islets (arrow); (g and h): G4 (STZ+5 % MC) showing increase number (arrows) and size of pancreatic islets and (i and j): G5 (STZ+10 % MC) showing alleviation of pancreatic lesions and restoring to the normal number (arrows) and size appearance of pancreatic tissue
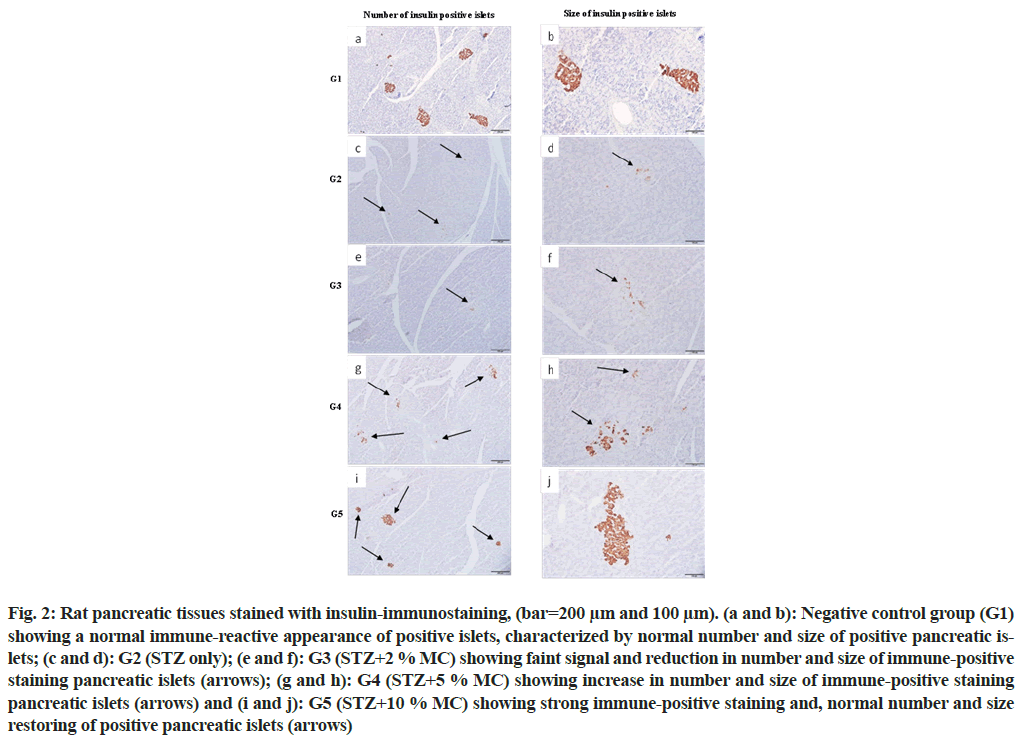
Strong insulin immuno-reactivity was observed in all pancreatic islets of the negative control group (G1), characterized by normal distribution with normal number and size of pancreatic islets (fig. 2a and fig. 2b), while, the positive control group (G2) revealed faint signal of insulin immuno-reactivity with few numbers and small size of pancreatic islets (fig. 2c and fig. 2d). In addition, rats that fed 2 % MC (G3), showed no significant differences compared with the positive control group (G2), (fig. 2e and fig. 2f). While, in groups G4 and G5, strong insulin immuno-reactivity with increase in the number and size of pancreatic islets were observed (fig. 2g-fig. 2j) respectively. The improvement in the number and size of insulin immuno-reactive pancreatic islets was more obvious in G5 which fed with 10 % MC.
Fig. 2: Rat pancreatic tissues stained with insulin-immunostaining, (bar=200 μm and 100 μm). (a and b): Negative control group (G1) showing a normal immune-reactive appearance of positive islets, characterized by normal number and size of positive pancreatic islets; (c and d): G2 (STZ only); (e and f): G3 (STZ+2 % MC) showing faint signal and reduction in number and size of immune-positive staining pancreatic islets (arrows); (g and h): G4 (STZ+5 % MC) showing increase in number and size of immune-positive staining pancreatic islets (arrows) and (i and j): G5 (STZ+10 % MC) showing strong immune-positive staining and, normal number and size restoring of positive pancreatic islets (arrows)
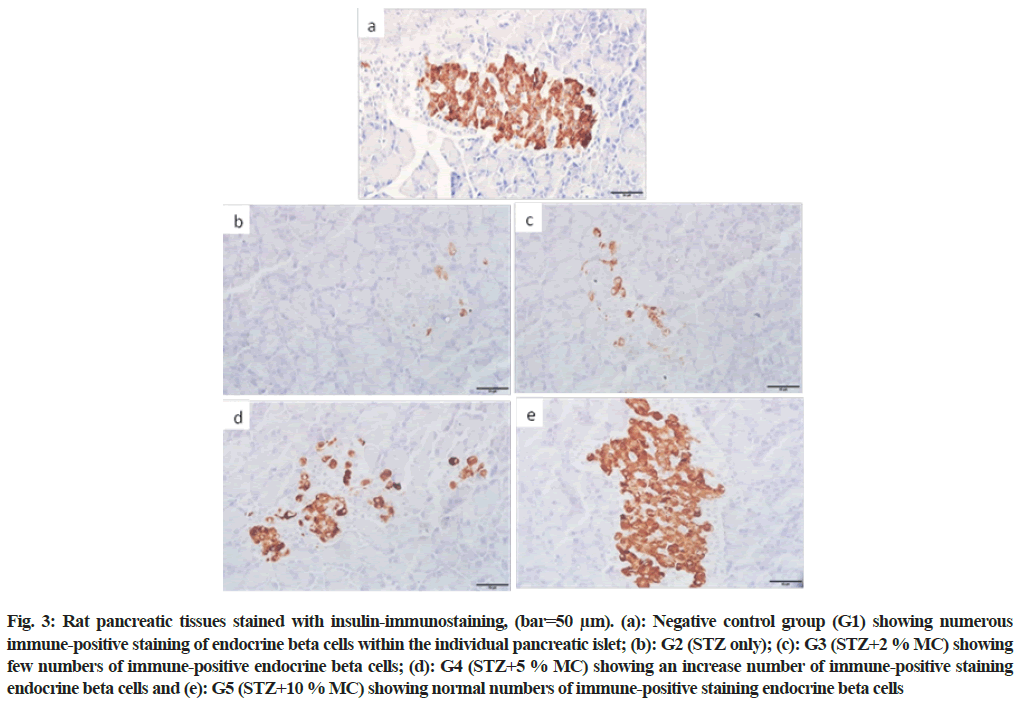
In the negative control group (G1), numerous normal insulin immuno-positive beta cells within all pancreatic islets were observed (fig. 3a). However, fewer insulin immuno-positive beta cells were seen in diabetic rats (G2) (fig. 3b). In group (G3), in spite of the rats fed 2 % MC, the insulin immuno-positive beta cells were still fewer compared to the control group (fig. 3c). Nevertheless, moderate restoring in the insulin immuno-positive beta cells were observed in group G4 (fig. 3d). While, group G5 showed numerous insulin immune-positive beta cells closely similar to the negative control group (fig. 3e).
Fig. 3: Rat pancreatic tissues stained with insulin-immunostaining, (bar=50 μm). (a): Negative control group (G1) showing numerous immune-positive staining of endocrine beta cells within the individual pancreatic islet; (b): G2 (STZ only); (c): G3 (STZ+2 % MC) showing few numbers of immune-positive endocrine beta cells; (d): G4 (STZ+5 % MC) showing an increase number of immune-positive staining endocrine beta cells and (e): G5 (STZ+10 % MC) showing normal numbers of immune-positive staining endocrine beta cells
As shown in Table 1, rats in (G1) showed normal blood glucose level, whereas, G2 showed a significant (p≤0.01) sharp increase in blood glucose level during each month along the experimental period. However G3, G4 and G5 showed gradual recovery represented by decreased glucose values in all treated groups related to the level of MC given and the duration of the experiment. In addition, there is a time-depended significant difference (p≤0.01) in the glucose parameters between rats treated with 2 %, 5 % and 10 % MC.
| Month | Groups | ||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |
| 1st mo | 128.20±2.195 | 504.00±13.832* | 471.63±13.30# | 390.89±18.979# | 347.11±11.569# |
| 2nd mo | 126.60±2.926 | 568.14±9.450* | 482.20±13.684# | 311.86±17.765# | 281.00±16.218# |
| 3rd mo | 126.40±2.202 | 579.20±9.521* | 480.20±12.644# | 262.67±12.170# | 192.43±8.693# |
Note: Data are represented as the mean±SE of values (n=10) and *p<0.01 represents significant difference in comparison to the control group. #p<0.01 represents significant variations compared to STZ alone and STZ with MC dose dependent treatment groups
Table 1: Effect of mc on Fasting Blood Glucose Levels (mg/dl) Each Month during the Experimental Period
DM is a chronic metabolic disorder caused by failure in any anti-hyperglycemic mechanisms includes insulin secretion from pancreatic beta cells. All antihyperglycemic drugs as well as exogenous insulin administration have been exhibiting several side effects[6]. Thus, several traditional approaches have been used to treat the DM. MC was promised traditional and medicinal plant that have antidiabetic effects via different mechanisms[29].
In the present study, the histopathological observations in negative control rats (G1) have shown a normal number, size and shape of pancreatic islets of Langerhans. Whereas, the positive control (diabetic control G2) showed atrophy in the size and reduction in the number of pancreatic islets and in addition, the destruction and disappearance of pancreatic islets cells. These results correspond with the findings of Abdollahi et al. and Hafizur et al.[30,31]. The effect of STZ at a single dose of 85 mg/kg body weight to rats is sufficient to induce multiple lesions in pancreatic islets related to T2DM. Meanwhile, all histopathological pancreatic lesions showed in G2 due to STZ injection were constant with the results obtained from study of Abdollahi et al. and Jin et al.[30,32]. However, all treated rats with 2 %, 5 % and 10 % MC (G3, G4 and G5) respectively, showed dose-dependent alleviation of pancreatic lesions caused by STZ-induced diabetes. It has been observed that the recovery of the islets is directly proportional to the dose of MC given to rats represented by mild restoring of pancreatic islets atrophy in G3 (2 % MC) and closely normal appearance of pancreatic islets, characterized by an increase in number and size of pancreatic islets in G4 and G5 (5 % and 10 % MC) respectively. These results were in parallel with the results obtained[11,30,31,33].
Previously, increase pancreatic islet size and number in STZ or alloxan-induced diabetic rats treated with MC have been noted[24,34,35]. Recently, Abdollahi et al.[30] suggested that MC may has a significant role in the renewal of pancreatic beta cells in the STZ rats by restoring degenerated mitochondrial structure via its antioxidant properties and increased the number of beta cells. Moreover, modulation of beta-cell function by increasing to almost double of islet size, total betacell area and number of beta cells were concluded[31,36]. However, this hypothesized mechanism has not been considered in studies[37,38].
Martinez et al.[39] suggested that there is a correlation between the decrease in hyperglycemia, the reduction of oxidative stress and the improvement of histopathological lesions. Moreover, Mahmoud et al. concluded that the MC pre-treatment before STZ induced diabetes protects the pancreatic tissues from injury due to antioxidants enhancement and reduction of lipid peroxidation. Hence, in our results, the improvement of pancreatic injury in MC treateddiabetic rats might be modulated due to the antioxidant effects of MC.
The present immunohistochemical observations revealed a normal topographical distribution involved normal number and size of insulin immuno-positive pancreatic islets and a normal number of insulin immuno-positive beta cells which is verified by strong insulin immune-reactivity in all negative control rats (G1). While, all positive control rats (G2) showed a reduced number and small size in positive pancreatic islets as well as few insulin immuno-positive beta cells verified by a faint signal of insulin immuno-reactive staining. These observations are harmonized with the results reported[33,40,41]. It has been reported that the dose of STZ injection is responsible for partial or total pancreatic beta-cell destruction results in insufficient insulin secretion that considered the most common reason of T2DM[27]. Thus, in our results the reduction of immuno-reactive staining of pancreatic islets in G2 is related to the STZ given to the rats that play important role in decreasing the number of beta cells and the number of insulin secretary granules. Nevertheless, rats fed 2 % MC (G3), have not showed any significant improvement in insulin immune-reactive pancreatic islets compared with the positive control group (G2). These results suggest that the MC dose (2 %) was not sufficient to alleviate the STZ effect and improve the number of beta cells as well as the number of insulin secretary granules. These observations are parallel with our previous observations[11]. However, G4 and G5 (5 % and 10 % MC respectively) showed strong variations in immune-positive signals of pancreatic islets and an increase in the number and size of insulin immunepositive pancreatic islets. Rats were fed with 5 % MC showed moderate restoring in the number of positive beta cells whereas, normal positive beta cells numbers, similar to the negative control group, were shown in rats fed with 10 % MC. These results agree with the results concluded[11,30,33,41].
It has been assumed that MC might have several mechanisms to ameliorate diabetic lesions and symptoms such as increase serum insulin levels and upregulates insulin gene in pancreatic beta cells[40,42], increase in adenosine monophosphate-activated protein kinase[43,44], glucose utilizing and up-regulation of Glucose Transporter 4 (GLUT4), Peroxisome Proliferator Activator Receptor Gamma (PPARγ) and Phosphatidylinositol-3 Kinase (PI3K)[45] and renewal, regeneration as well as a proliferation of surviving pancreatic beta cells[24]. In addition, several studies have demonstrated that MC possesses different ingredients appear to have animal insulin-like polypeptides such as lecithin, charantin and triterpenoids responsible for augmentation of glucose as well as amino acid uptake into skeletal muscle cells[24,46]. Therefore, our immunohistological results confirmed that MC has improved mechanism against pancreatic injury via renewal, regeneration as well as proliferation of surviving insulin immune-positive pancreatic beta cells and subsequently ameliorate insulin secretion.
The histopathological and immunohistochemical observations of the present study were confirmed by measuring the blood glucose levels, where the rats that given only STZ (G2) showed a sharp increase in blood glucose level (hyperglycemia) compared to normal control (G1) during every month along the experimental period. However, the rats which were treated with 2 %, 5 % and 10 % MC (G3, G4 and G5) after STZ injection showed gradual dose and time-dependent recovery represented by decreased glucose values during each month along the experimental period. This finding is consistent with the results obtained[11,35,47].
Any impairment in insulin regulation, secretion, uptake or breakdown leads to hyperglycemia. On the other hand, any pancreatic beta cells damage will lead to persistent hyperglycemia[48]. Our findings confirmed that MC increased insulin secretion by increasing the number and size of insulin immuno-positive pancreatic islets and beta cells subsequently reduce the fasting serum glucose in diabetic rats through 12 w treatment. Increase insulin secretion exerts the body cells to utilize blood glucose and maintaining blood hyperglycemia. In addition, MC may be responsible for ameliorating insulin resistance. It has been reported that MC treatment increases GLUT-4 protein in the plasma membrane[49], increases hepatic glycogenesis and reduced glycolysis[50] and improves insulin sensitivity[42].
In conclusion, these results confirmed that MC might have a role in the renewal of pancreatic beta cells by increasing the number of beta cells and the number of insulin secretary granules in STZ-induced diabetic rats, subsequently promoting insulin secretion and controlling hyperglycemia.
Aknowlegment:
The authors acknowledge the Deanship of Scientific Research at King Faisal University for the financial support under Nasher Track (Grant No. 206050).
Conflict of interests:
The authors declared no conflict of interests.
References
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271-81.
[Crossref] [Google Scholar] [PubMed]
- Kumar P, Clark M. Textbook of clinical medicine. 8th ed. Pub: Saunders, London; 2002. p. 1099-121.
- Global report on diabetes. World Health Organization; 2016.
- Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med 2011;31(1):19-23.
[Crossref] [Google Scholar] [PubMed]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414(6865):799-806.
[Crossref] [Google Scholar] [PubMed]
- Holman RR, Turner RC. Oral agents and insulin in the treatment of NIDDM. Textbook of Diabetes. Oxford: Blackwell. 1991;9:467-9.
- Yin RV, Lee NC, Hirpara H, Phung OJ. The effect of bitter melon (Mormordica charantia) in patients with diabetes mellitus: A systematic review and meta-analysis. Nutr Diabetes 2014;4(12):e145.
[Crossref] [Google Scholar] [PubMed]
- Win MT. Novel effect of medicinal plants on diabetes mellitus. J Diab Metab Disorder Control 2020;7(2):73-4.
- Chekka SV, Mantipelly NK. Momordica charantia: A natural medicinal plant. GSC Biol Pharm Sci 2020;12(2):129-35.
- Xu X, Shan B, Liao CH, Xie JH, Wen PW, Shi JY. Anti-diabetic properties of Momordica charantia L. polysaccharide in alloxan-induced diabetic mice. Int J Biol macromol 2015;81:538-43.
[Crossref] [Google Scholar] [PubMed]
- Moqbel MS, Al-Hizab FA, Barakat SM. Clinicopathological study on the effects of Momordica charantia on Streptozotocin-induced diabetic Wistar rats. Open J Vet Med 2017;7(5):49.
- Akhter R, Rasel IH, Islam MS. Antidiabetic effect of bitter melon/Kerala (Momordica charantia) in alloxan induced diabetic rat. Res Agric Livest Fish 2018;5(3):373-9.
- Ooi CP, Yassin Z, Hamid TA. Momordica charantia for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012;8:CD007845.
[Crossref] [Google Scholar] [PubMed]
- Rahman IU, Khan RU, Rahman KU, Bashir M. Lower hypoglycemic but higher antiatherogenic effects of bitter melon than glibenclamide in type 2 diabetic patients. Nutr J 2015;14(1):1-13.
[Crossref] [Google Scholar] [PubMed]
- Manabe M, Takenaka R, Nakasa T, Okinaka O. Induction of anti-inflammatory responses by dietary Momordica charantia L. (bitter gourd). Biosci Biotechnol Biochem 2003;67(12):2512-7.
[Crossref] [Google Scholar] [PubMed]
- Nerurkar P, Ray RB. Bitter melon: Antagonist to cancer. Pharm Res 2010;27(6):1049-53.
[Crossref] [Google Scholar] [PubMed]
- Alam S, Asad M, Asdaq SM, Prasad VS. Antiulcer activity of methanolic extract of Momordica charantia L. in rats. J Ethnopharmacol 2009;123(3):464-9.
[Crossref] [Google Scholar] [PubMed]
- Shan B, Xie JH, Zhu JH, Peng Y. Ethanol modified supercritical carbon dioxide extraction of flavonoids from Momordica charantia L. and its antioxidant activity. Food Bioprod Process 2012;90(3):579-87.
- Yang ZG, Shi WZ, Shen ZG, Zhang YP, Yang J. Preventive effect of Momordica charantia L. saponins on osteoporosis in ovariectomized rats. Food Sci 2010;31(7):272-5.
- Ramalhete C, Mulhovo S, Molnar J, Ferreira MJ. Triterpenoids from Momordica balsamina: Reversal of ABCB1-mediated multidrug resistance. Bioorg Med Chem 2016;24(21):5061-7.
[Crossref] [Google Scholar] [PubMed]
- Wang Q, Wu X, Shi F, Liu Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed Pharmacother 2019;109:744-50.
[Crossref] [Google Scholar] [PubMed]
- Zhang C, Chen H, Bai W. Characterization of Momordica charantia L. polysaccharide and its protective effect on pancreatic cells injury in STZ-induced diabetic mice. Int J Biol Macromol 2018;115:45-52.
[Crossref] [Google Scholar] [PubMed]
- Jia S, Shen M, Zhang F, Xie J. Recent advances in Momordica charantia: Functional components and biological activities. Int J Mol Sci 2017;18(12):2555.
[Crossref] [Google Scholar] [PubMed]
- Ahmed I, Adeghate E, Cummings E, Sharma AK, Singh J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol Cell Biochem 2004;261(1):63-70.
[Crossref] [Google Scholar] [PubMed]
- Pahlavani N, Roudi F, Zakerian M, Ferns GA, Navashenaq JG, Mashkouri A, et al. Possible molecular mechanisms of glucose-lowering activities of Momordica charantia (karela) in diabetes. J Cell Biochem 2019;120(7):10921-9.
[Crossref] [Google Scholar] [PubMed]
- Shih CC, Lin CH, Lin WL, Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol 2009;123(1):82-90.
[Crossref] [Google Scholar] [PubMed]
- Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70(1):5-47.
[Crossref] [Google Scholar] [PubMed]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Elsevier health sciences. China: Churchill Livingstone; 2008.
- Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): A mini review. Br J Nutr 2009;102(12):1703-8.
[Crossref] [Google Scholar] [PubMed]
- Abdollahi M, Zuki AB, Goh YM, Rezaeizadeh A, Noordin MM. Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol Histopathol 2011;26(1):13-21.
[Crossref] [Google Scholar] [PubMed]
- Hafizur RM, Kabir N, Chishti S. Modulation of pancreatic β-cells in neonatally streptozotocin-induced type 2 diabetic rats by the ethanolic extract of Momordica charantia fruit pulp. Nat Prod Res 2011;25(4):353-67.
[Crossref] [Google Scholar] [PubMed]
- Jin X, Zeng L, He S, Chen Y, Tian B, Mai G, et al. Comparison of single high-dose streptozotocin with partial pancreatectomy combined with low-dose streptozotocin for diabetes induction in rhesus monkeys. Exp Biol Med 2010;235(7):877-85.
[Crossref] [Google Scholar] [PubMed]
- Ahmed I, Adeghate E, Sharma AK, Pallot DJ, Singh J. Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes Res Clin Pract 1998;40(3):145-51.
[Crossref] [Google Scholar] [PubMed]
- Singh N, Gupta M. Regeneration of β cells in islets of langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian J Exp Biol 2007;45(12):1055-62.
[Google Scholar] [PubMed]
- Mahmoud MF, El Ashry FE, El Maraghy NN, Fahmy A. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm Biol 2017;55(1):758-65.
[Crossref] [Google Scholar] [PubMed]
- Ketut DM, Adnyana DP, Rinaldhi CP, Octaviani RR, Cempaka DK. The antidiabetic effect of bitter melon (Momordica charantia L.) extracts towards glucose concentration, langerhans islets and leydig cells of hyperglycemic mice (Rattus norvegicus). EurAsian J BioSci 2019;13(2).
- Day C, Cartwright T, Provost J, Bailey CJ. Hypoglycaemic effect of Momordica charantia extracts. Planta Med 1990;56(5):426-9.
[Crossref] [Google Scholar] [PubMed]
- Sarkar S, Pranava M, Marita AR. Demonstration of the hypoglycemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol Res 1996;33(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Martinez G, Al-Dalain SM, Menéndez S, Guiliani A, León OS. Ozone treatment reduces blood oxidative stress and pancreas damage in a streptozotocin-induced diabetes model in rats. Acta Farm Bonaerense 2005;44(5):491-7.
- Malekshahi H, Bahrami G, Miraghaee S, Ahmadi SA, Sajadimajd S, Hatami R, et al. Momordica charantia reverses type II diabetes in rat. J Food Biochem 2019;43(11):e13021.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Rahman RF, Soliman GA, Saeedan AS, Ogaly HA, Abd-Elsalam RM, Alqasoumi SI, et al. Molecular and biochemical monitoring of the possible herb-drug interaction between Momordica charantia extract and glibenclamide in diabetic rats. Saudi Pharm J 2019;27(6):803-16.
[Crossref] [Google Scholar] [PubMed]
- Ma C, Yu H, Xiao Y, Wang H. Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharm Biol 2017;55(1):2170-7.
- Chaturvedi P. Antidiabetic potentials of Momordica charantia: Multiple mechanisms behind the effects. J Med Food 2012;15(2):101-7.
[Crossref] [Google Scholar] [PubMed]
- McCarty MF. Does bitter melon contain an activator of AMP-activated kinase? Med Hypotheses 2004;63(2):340-3.
[Crossref] [Google Scholar] [PubMed]
- Kumar R, Balaji S, Uma TS, Sehgal PK. Fruit extracts of Momordica charantia potentiate glucose uptake and up-regulate Glut-4, PPARγ and PI3K. J Ethnopharmacol 2009;126(3):533-7.
[Crossref] [Google Scholar] [PubMed]
- Han JH, Tuan NQ, Park MH, Quan KT, Oh J, Heo KS, et al. Cucurbitane triterpenoids from the fruits of Momordica charantia improve insulin sensitivity and glucose homeostasis in streptozotocin-induced diabetic mice. Mol Nutr Food Res 2018;62(7):1700769.
[Crossref] [Google Scholar] [PubMed]
- Cortez-Navarrete M, Martinez-Abundis E, Perez-Rubio KG, Gonzalez-Ortiz M, Méndez-del Villar M. Momordica charantia administration improves insulin secretion in type 2 diabetes mellitus. J Med Food 2018;21(7):672-7.
[Crossref] [Google Scholar] [PubMed]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001;50(6):537-46.
[Google Scholar] [PubMed]
- Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, et al. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutr Sci Vitaminol 2001;47(5):340-4.
[Crossref] [Google Scholar] [PubMed]
- Wahren J, Ekberg K. Splanchnic regulation of glucose production. Ann Rev Nutr 2007;27:329-45.
[Crossref] [Google Scholar] [PubMed]