- *Corresponding Author:
- Hamid
Department of Food Technology and Nutrition,
School of Agriculture,
Lovely Professional University,
Phagwara,
Punjab 144411
E-mail: hamidfst6789@gmail.com
| Date of Received | 03 March 2020 |
| Date of Revision | 26 November 2021 |
| Date of Acceptance | 07 July 2022 |
| Indian J Pharm Sci 2022;84(4):838-847 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study wild pomegranate flavedo powder was used for the extraction of phenolics through various solvents by reflux method. A significant difference in all parameters was observed among the different extracts with varying solvents and extraction time after oven drying. The highest total phenolics, total flavonoids, 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity, ferric reducing antioxidant power and metal chelating activity among different extracts of wild pomegranate flavedo were recorded when combination of ethanol and distilled water in the ratio of 50:50 was used (1 h extraction time) after oven drying. Cluster analysis based on Ward’s method showed that all treatments were divided in nine major groups and three major clusters after the application of cluster analysis. The two best selected extracts were further compared after drying in lyophilizer and tested against Staphylococcus aureus and Escherichia coli for antibacterial activity. In conclusion, ethanol and distilled water lyophilized extract contained higher bioactive compounds than oven dried extract, which have higher antioxidant and antibacterial effects and could be useful in the extraction of natural antioxidants. The study concluded that fruit flavedo is rich source of total phenolics, total flavonoids and antioxidant activity which have beneficial natural antioxidants for health and have many health promoting properties which makes the flavedo suitable for future processing into various functional/nutraceutical foods.
Keywords
Natural antioxidants, ferric reducing antioxidant power, phenolics, wild pomegranate flavedo powder, antibacterial effects
Wild pomegranate (Punica granatum L.) belongs to the family Punicaceae which is an important wild fruit with great economic significance. In India, it is found in vast tract of the hill slopes of Himachal Pradesh, Jammu and Kashmir and Uttarakhand at an altitude of 900 to 1800 m above mean sea level. Wild pomegranate fruits have been known for thick rinds and higher acidity than commercial ones[1]. In recent years, the demand for its value-added products got increased due to its recognition as a great source of natural antioxidants and health promoting constituents like organic acids, anthocyanins, phenolics, vitamins and minerals[2,3]. Its fruit constitute three portions like albedo, flavedo and arils. Arils are rich source of organic acids apart from having appreciable amount of sugars, minerals and antioxidants like anthocyanins, phenols, ascorbic acid etc.[4,5], however flavedo portion of this fruit which constitute 50 % of the fruit is rich source of natural polyphenolic antioxidants like gallic acid, quercetin, ellagic acid and punicalagin[6]. Different parts of commercial pomegranate fruit contains different bioactive polyphenolic compounds, therefore whole fruit extract can be the interesting dietary supplement and nutraceutical[7,8]. Polyphenols mainly ellagitannins and ellagic acid are abundant in pomegranate peel and responsible for the antioxidant, antimutagenic, anti- cancer, anti-inflammatory and anti-diabetic effects[9]. However, the reciprocal concentration/bioavailability of these antioxidant compounds may vary depending upon cultivar type, region and various development phases of the fruit. The attention towards antioxidants from plant based sources (natural) for protection against various diseases induced by free radicals and their controlled applications in functional food products development likely to generate beneficial health effects[10]. In the present study, the extraction conditions of phenolic compounds from wild pomegranate flavedo were standardized and their antioxidant activities of various types of solvent extracts were investigated using reflux method. Antimicrobial activity of extracts after lyophilization at different concentrations was also examined and a relationship was established between these activities and the phenolics and flavonoids contents. So that these type of extracts were used in supplementation of food with bioactive compounds as well as helps in waste reduction.
Materials and Methods
Wild pomegranate fruits were procured at optimum maturity from Karsog location of Himachal Pradesh in the month of October 2017. After harvesting, fruits were transported to the laboratory through a well- ventilated vehicle on the same day and stored at 0° till analysis. The flavedo from fruits were further separated and dried in mechanical cabinet drier (50°)[11].
Extraction:
The mechanical dried flavedo was further utilized for making flavedo powder by pulverizer having particle size of 425 microns through 36 mesh metallic sieve. The prepared flavedo powder was further used for extraction of phenolics through reflux method (Table 1). In this method of extraction, constant solid to solvent ratio (1:20) was refluxed under heat with varying extraction solvents and time. For each treatment 10 g of flavedo powder was dissolved in 200 ml of respective solvent followed by refluxing under heat for different time periods. The extract obtained was further concentrated in vacuum rotatory evaporator (at 50°) until 1/4th of the initial volume remained followed by oven drying (50°) of extract. The best treatment with higher phenolic content were further freeze-dried using a lyophilizer (Labconco-FreeZone United States of America (USA)) at a constant temperature of -30° with 0.04 mbar vacuum pressure upto a constant weight[6]. The chemicals used during the entire study were of analytical grade and reference standard gallic acid and quercetin were of Sigma-Aldrich.
| S. No. | Solvents | Extraction time (h) | ||
|---|---|---|---|---|
| 1 | Distilled water | 1 | 2 | 3 |
| 2 | Ethanol | 1 | 2 | 3 |
| 3 | Ethanol:Distilled water (80:20) | 1 | 2 | 3 |
| 4 | Ethanol:Distilled water (60:40) | 1 | 2 | 3 |
| 5 | Ethanol:Distilled water (50:50) | 1 | 2 | 3 |
| 6 | Acetone | 1 | 2 | 3 |
| 7 | Acetone:Distilled water (50:50) | 1 | 2 | 3 |
| 8 | Ethanol:Acetone:Distilled water (1:1:1) | 1 | 2 | 3 |
| 9 | Ethyl acetate | 1 | 2 | 3 |
| 10 | Ethanol:Diethyl ether:Distilled water (80:10:10) | 1 | 2 | 3 |
| 11 | Diethyl ether | 1 | 2 | 3 |
Table 1: Details of Treatments for Extraction of Phenolics by Reflux Method
Physical characteristics:
Colour of samples was measured by a Lovibond colour Tintometer Model PFXi series spectrophotometer in which RYBN colour units were obtained along with Common Intellectual Experience (CIE) readings i.e. L*, a* and b* values. The L* value gives a measure of the lightness of the product colour from 100 to 0, 100 for perfect white while 0 for black. The a* value represents the green to red colour range and b* values represents yellow to blue colour range[6]. The time taken to dry a given tray load was calculated by recording the time (h) required by the material in the tray till it attains a constant weight after drying in respective drying modes. Total extract yield was calculated by dividing the weight of dried material by the weight of fresh material multiplying by 100.
Antioxidants and antioxidant properties:
Total phenolic content was determined by Folin- Ciocalteu procedure given by Singleton and Rossi[12] in which absorbance was measured at 765 nm in a spectrophotometer (Model Ultraviolet (UV)-1650 PC Shimadzu, Japan) against water blank. A standard calibration curve of gallic acid (20 to 100 µg/ml) using its different concentrations was prepared. Total flavonoid content was estimated according to the method of Ilahy et al.[13]. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging activity was measured as per the method of Brand-Williams et al.[14]. Reducing power was determined as per the method of Oktay et al.[15] and absorbance of the sample extract at 700 nm was taken as a measure of reducing power. 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1 % potassium ferricyanide were added to 0.1 ml of methanolic (1 ml/g sample in 10 ml methanol) extract. The combination of 25 ml of 10 % trichloroacetic acid was then incubated at 50° for 20 min. 2.5 ml of supernatant was obtained after adding acid and centrifuging at 3000 rpm for 10 min. 2.5 ml of distilled water and 0.5 ml of a solution of 0.1 % Ferric chloride (FeCl3) was added. The sample’s absorbance in UV-Vis spectrophotometer (photometer) (Model UV-1650 PC Shimadzu, Japan), at 700 nm was used as a measure of reducing power. Metal chelating activity was determined according to method of Dinis et al.[16] and Ferric Reducing Antioxidant Power (FRAP) of the samples was estimated according to the method of Benzie and Strain[17].
Antimicrobial activity:
Antimicrobial activity of selected phenolic extracts against two test microorganisms i.e. Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) was detected by well diffusion method. A loopful culture of the test microorganisms was inoculated into 100 ml of nutrient broth in 250 ml Erlenmeyer flask. The test microorganisms were first grown in nutrient broth for 24-36 h at 37°. Wells of 6 mm diameter were cut into prepoured, sterilized nutrient agar petriplates with a sharp and sterile borer. Lawn of respective test microorganism to be tested against the different phenolics extracts on these petriplates was prepared by pouring 0.1 ml of inoculum and swabbing it properly with the help of sterilized cotton buds in such a way that test microorganism cover whole of the nutrient agar plate. After that extracts of different concentrations 25, 50, 75 and 100 ppm were prepared by dissolving phenolics extract powder in distilled water and keeping it overnight. After extract preparation different extracts were filled in already prepared 6 mm wells under laminar air flow. Then petri-plates were incubated at 37° for 24 h and results obtained were expressed in the form of zone of inhibition (mm). The diameter of inhibition zone formed by extracts against the respective test microorganism was measured. In the same way, all the samples of phenolic extracts were tested against each test microorganisms[6].
Statistical analysis:
The data on physico-chemical characteristics of extracts were analyzed by the Completely Randomized Design (CRD). One way Analysis of Variance (ANOVA) with (p<0.05) was used to analyze significant differences between treatments. Cluster analysis was performed by using ward method (Statistical Package for the Social Sciences (SPSS)). Different treatments of extraction by reflux method were grouped into cluster form on the basis of similarities among total extract yield, total phenolic content and total flavonoid content of respective solvents extract.
Results and Discussion
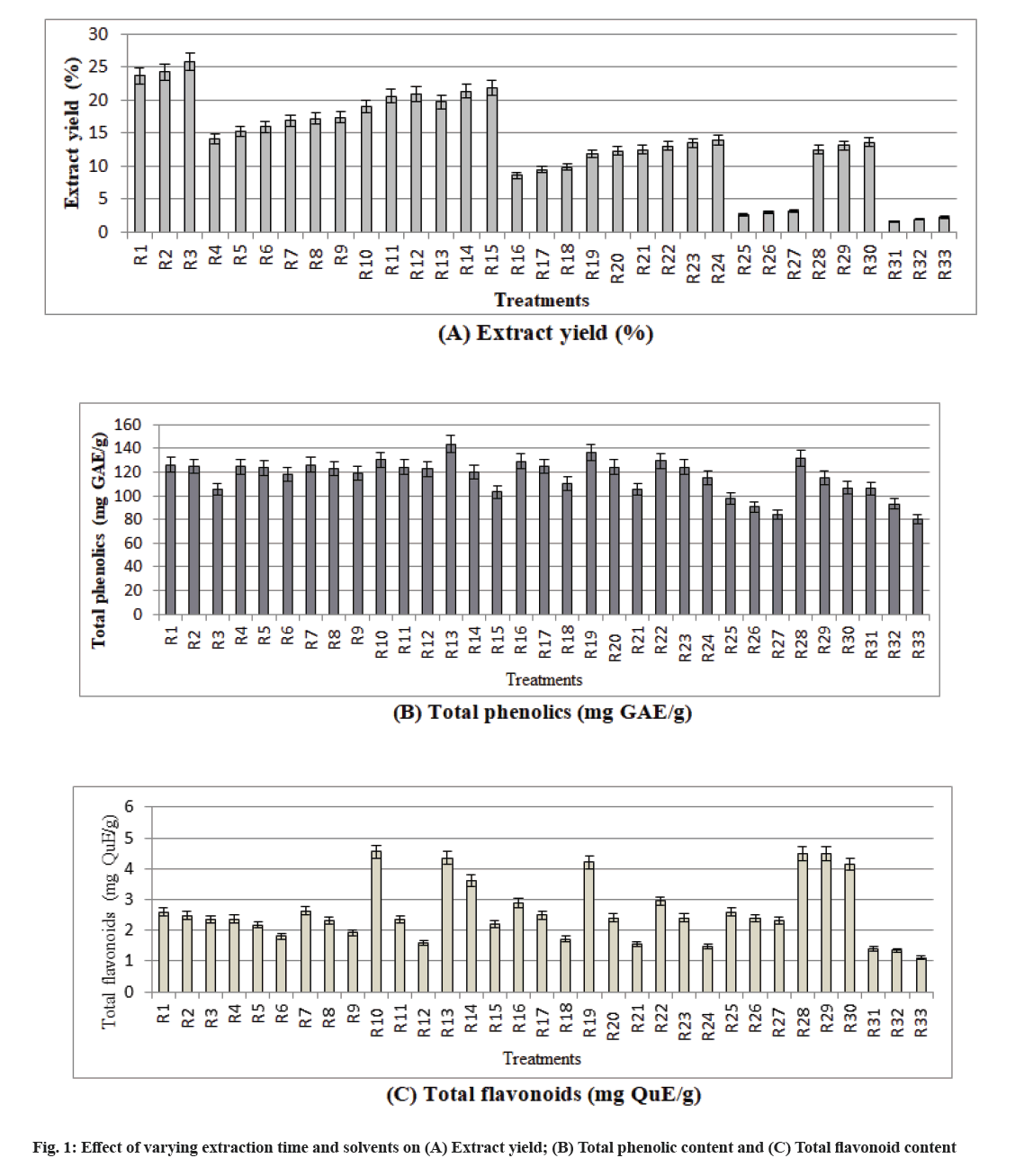
The effect of extraction solvent and time on the extract yield, total phenolics and flavonoids of wild pomegranate flavedo extract has been highlighted in fig. 1A-fig. C, Table 2 and Table 3. A significant effect of extraction solvent and time on all the parameters was observed with varying solvent types.
| Parameters | Treatments | Drying modes | |
|---|---|---|---|
| D1 | D2 | ||
| Extract yield (%) | T1 | 19.70±0.7a | 21.66±0.20 a |
| T2 | 11.86±0.14b | 12.05±0.47b | |
| Total phenolics (mg GAE/g) | T1 | 143.59±0.57a | 158.76±0.80a |
| T2 | 136.21±0.79b | 152.49±1.02b | |
| Total flavonoids (mg QuE/g) | T1 | 4.35±0.50a | 7.90±0.78a |
| T2 | 4.22±0.10b | 6.53±0.97b | |
Note: Different superscripts letters a,b in the same column indicate significant differences (p<0.05). T1: Ethanol and distilled water (50:50) extract after 1 h of extraction by reflux method; T2: Acetone and distilled water (50:50) extract after 1 h of extraction by reflux method; D1: Oven dried phenolic extract powder and D2: Lyophilized phenolic extract powder
Table 2: Extract Yield, Total Phenolics and Total Flavonoids of Oven Dried and Lyophilized Phenolic Extract Powder of Different Treatments
In fig. 1 it is shown that, R1: Distilled water (1 h), R2: Distilled water (2 h), R3: Distilled water (3 h), R4: Ethanol (1 h), R5: Ethanol (2 h), R6: Ethanol (3 h), R7: Ethanol:Distilled water (80:20) (1 h), R8: Ethanol:Distilled water (80:20) (2 h), R9: Ethanol:Distilled water (80:20) (3 h), R10: Ethanol:Distilled water (60:40) (1 h), R11: Ethanol:Distilled water (60:40) (2 h), R12: Ethanol:Distilled water (60:40) (3 h), R13: Ethanol:Distilled water (50:50) (1 h), R14: Ethanol:Distilled water (50:50) (2 h), R15: Ethanol:Distilled water (50:50) (3 h), R16: Acetone (1 h), R17: Acetone (2 h), R18: Acetone (3 h), R19: Acetone:Distilled water (50:50) (1 h), R20: Acetone:Distilled water (50:50) (2 h), R21: Acetone:Distilled water (50:50) (3 h), R22: Ethanol:Acetone:Distilled water (1:1:1) (1 h), R23: Ethanol:Acetone:Distilled water (1:1:1) (2 h), R24: Ethanol:Acetone:Distilled water (1:1:1) (3 h), R25: Ethyl acetate (1 h), R26: Ethyl acetate (2 h), R27: Ethyl acetate (3 h), R28: Ethanol:Diethyl ether:Distilled water (80:10:10) (1 h), R29: Ethanol:Diethyl ether:Distilled water (80:10:10) (2 h), R30: Ethanol:Diethyl ether:Distilled water (80:10:10) (3 h), R31: Diethyl ether (1 h), R32: Diethyl ether (2 h), R33: Diethyl ether (3 h).
Fig. 1A shows that with increase in extraction time from 1 to 3 h, extract yield increased from 23.65 % to 25.77 % (distilled water), 14.08 % to 15.90 % (ethanol), 16.88 % to 17.36 % [combination of ethanol and distilled water (80:20)], 19.02 % to 20.90 % (combination of ethanol and distilled water in the ratio of 60:40), 19.70 % to 21.85 % (combination of ethanol and distilled water in the ratio of 50:50), 8.57 % to 9.78 % (acetone), 11.86 % to 12.46 % (combination of acetone and distilled water in the ratio of 50:50), 13.00 % to 13.88 % [combination of ethanol, acetone and distilled water (1:1:1)], 2.55 % to 3.20 % (ethyl acetate), 12.46 % to 13.56 % [combination of ethanol, diethyl ether and distilled water (80:10:10)] and 1.55 % to 2.20 % was observed, when diethyl ether was used as a solvent. While comparing all the treatments, the extract yield increased with the increase in extraction time in all the solvents. However, the maximum extract yield (25.77 %) was observed in distilled water (R3) after 3 h of extraction and minimum (1.55 %) in diethyl ether (R31) after 1 h of extraction. Highest extract yield with distilled water recorded at maximum extraction time (3 h) might be due to its polar nature coupled with prolonged extraction time which increased the efficiency of the extraction since heat render the cell walls more permeable, increase solubility and diffusion coefficients of the compounds to be extracted and decreases the viscosity of the solvent, thus facilitated its passage through the solid substrate mass. Other reason of higher yield of the extract might be due to the higher amount of proteins and carbohydrates extraction because of their more solubility in water than in ethanol and acetone[18]. The lower extract yield observed with diethyl ether might be due to non-polar nature of the solvent as compared to other solvents which led to lower extract yield of polar compounds. Higher extract yield observed after 3 h of extraction with all the solvents might be due to the prolonged extraction time which increased the efficiency of the extraction but also equilibrium attained as very small increase in extraction achieved as compared to 1 h of extraction. However, as distilled water and ethanol are polar protic solvents with high dielectric constant and higher dipole moment, it facilitated the extraction of higher amount of phenolics as compared to polar aprotic solvents (acetone and ethyl acetate).
The data presented in fig. 1B indicate that total phenolic content decreased with the increase in extraction time (from 1 to 3 h) from 125.97 to 105.46 mg Gallic Acid Equivalents (GAE)/g (distilled water), 124.66 to 118.03 mg GAE/g (ethanol), 126.32 to 119.38 mg GAE/g (combination of ethanol and distilled water in the ratio of 80:20), 130.50 to 122.70 mg GAE/g (combination of ethanol and distilled water in the ratio of 60:40), 143.59 to 103.13 mg GAE/g (combination of ethanol and distilled water in the ratio 50:50), 128.98 to 110.24 mg GAE/g (acetone), 136.21 to 105.52 mg GAE/g (combination of acetone and distilled water in the ratio of 50:50), 129.50 to 114.98 mg GAE/g (combination of ethanol, acetone and distilled water in the ratio of 1:1:1), 98.10 to 84.17 mg GAE/g (ethyl acetate), 131.53 to 106.95 mg GAE/g (combination of ethanol, diethyl ether and distilled water in the ratio of 80:10:10), 106.06 to 80.05 mg GAE/g (diethyl ether).
While comparing all the treatments, the maximum total phenolic content as 143.59 mg GAE/g was observed in combination (R13) of ethanol and distilled water (50:50) after 1 h of extraction and minimum total phenolic content as 80.05 mg GAE/g was observed in diethyl ether (R33) after 3 h of extraction. The polarities of the solvents range from polar to non-polar and optimum extraction of polyphenols is usually obtained in the polar solvent which have a better efficiency of solvation as a result of hydrogen bonds interactions between the polar sites of the antioxidant compounds[19]. The increase in solubility with the addition of water to organic solvents is due to the weakening of the hydrogen bonds in aqueous solutions and increase of ionization of the polyphenols in such solutions[20]. This might be the reason of higher extraction of phenolic compounds in combination of ethanol and water as a solvent. While lower phenolic content observed in diethyl ether might be due to the non-polar nature of this solvent which led to lower extraction of polar phenolic antioxidants because of lower capability to break covalent molecules into ions. The comparable total phenolic content observed after 1 h of extraction in all the solvents might be due to lower amount of degradation of the phenolics at this extraction time. Thermal processing for longer duration (3 h) at boiling point of solvent might also result in the loss of natural antioxidants because heat accelerates their oxidation and other degenerative reactions[21].
An appraisal of data given in fig. 1C indicates that with increase in extraction time from 1 to 3 h, total flavonoid content decreased from 2.59 to 2.35 mg Quercetin Equivalent (QuE)/g (distilled water), 2.37 to 1.80 mg QuE/g (ethanol), 2.63 to 1.93 mg QuE/g (combination of ethanol and distilled water 80:20), 4.55 to 1.59 mg QuE/g (combination of ethanol and distilled water 60:40), 4.35 to 2.21 mg QuE/g (ethanol and distilled water 50:50), 2.89 to 1.72 mg QuE/g (acetone), 4.22 to 1.55 mg QuE/g (acetone and distilled water 50:50), 2.95 to 1.49 mg QuE/g (ethanol, acetone and distilled water 1:1:1), 2.60 to 2.30 mg QuE/g (ethyl acetate), 4.49 to 4.15 mg QuE/g (ethanol, diethyl ether and distilled water 80:10:10) and 1.41 to 1.10 mg QuE/g when diethyl ether was used as a solvent. While comparing all the treatments, the total flavonoid content decreased with the increase in extraction time in all the solvents. However, maximum total flavonoid content as 4.55 mg QuE/g was observed in the combination (R10) of ethanol and distilled water (60:40) as solvent after 1 h of extraction and minimum content as 1.10 mg QuE/g was observed in diethyl ether (R33) after 3 h of extraction. Highest total flavonoid content observed after 1 h of extraction with the combination of ethanol and distilled water (60:40) might be due to the increased polarity of ethanol as a result of addition of water to it which facilitated the extraction of higher amount of antioxidant compounds. The increase in solubility upon the addition of water to organic solvents could be due to the weakening of the hydrogen bonds in aqueous solutions and increase of ionization of the polyphenols in such solutions. Our findings are consistent with those of Musa et al.[22]. Whereas, minimum total flavonoid content in diethyl ether might be due to the lower polarity because of its lower capability to break covalent molecules into ions[23].
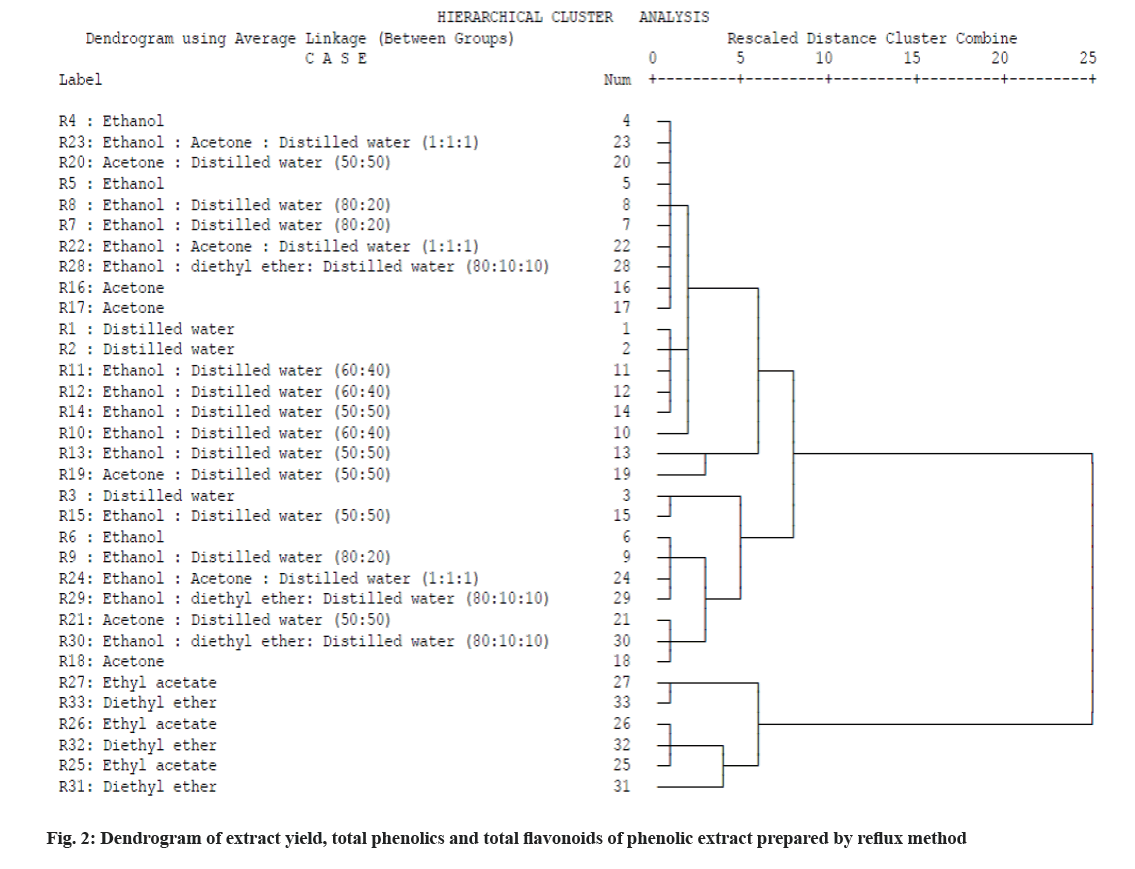
In cluster analysis or hierarchical clustering, samples are grouped on the basis of similarities without taking into account the information about the class membership. Cluster analysis calculates the distances (or correlation) between all samples using a defined metric[24]. As here in this study the number of treatments is greater than 30 so applied cluster analysis unveil how many similar outcomes are there from different treatments as they identified by this technique and categorized into groups on the basis of similar phenolic content and flavonoids content after extraction. Also cluster analysis in this study clearly reveals the differences in the extraction patterns of the bioactive compounds with respect to time and solvents. So cluster analysis of phenolic extract of 33 treatments was performed using rescaled distance cluster with respect to three parameters (total extract yield, total phenolics and total flavonoids). The results obtained from cluster analysis are shown as a dendrogram in fig. 2. All treatments were divided in nine major groups and three major clusters after the application of cluster analysis. The first group 1 was composed of treatments R4, R23, R20, R5, R8, R7, R22, R28, R16, R17; in group 2, R1, R2, R11, R12, R14, R10; in group 3, R13, R19; in group 4, R3, R15; in group 5, R6, R9, R24, R29; in group 6; R21, R30, R18; in group 7, R27, R33; in group 8, R26, R32, R25 and in group 9, treatment R9. It is evident from the obtained dendrogram in fig. 2 that the samples were distributed among different well-defined clusters based on their similarity. The same treatments (R13 and R19) with higher phenolic content as mentioned in fig. 1 are positioned in the same group and in same cluster based upon similarity. So, on the basis of hierarchical clustering of the data of extract yield, total phenolics and total flavonoids of wild pomegranate flavedo extract, two treatments (R13 and R19) from cluster (1) were selected for further studies due to interlinked similarities between these two treatments. Similarly in previous studies, the hierarchical clustering was applied to show similar characteristics of different treatments[23].
Comparison of oven and freeze dried phenolic extract powder of wild pomegranate flavedo is described here. The two best selected treatments from fig. 1A-fig. 1C was further compared on the basis of extract yield, total phenolic and flavonoid content. Data in Table 2 highlights that significant differences were observed in drying time among both the treatments while comparing the oven dried (4.50 h and 0.50 h) and lyophilized phenolic extract powder (53.20 h and 31.20 h) of wild pomegranate flavedo. It took more time to dry the phenolic extract in lyophilizer as compared to oven. Similarly, in oven dried extracts the maximum drying time was recorded as 4.50 h in treatment T1. Data in Table 2 show overall higher extract yield was observed in lyophilized samples of all the treatments as compared to oven dried samples. However, the maximum extract yield was recorded by lyophilization as 21.66 % in treatment T1. Similarly, the maximum extract yield was recorded after oven drying as 19.70 % in treatment T1. Data in Table 2 reflect that total phenolic content and total flavonoid content were recorded higher in lyophilized samples of all the treatments as compared to oven dried samples. The maximum amount of phenolics and flavonoids in freeze dried extract might be due to the better stability of various antioxidant compounds at low temperature as compared to high drying temperature as reported in earlier study by Hamid et al.[11] temperature significantly affects the retention of bioactive compounds. Our results are within the range of phenolics reported by Azarpazhooh et al.[25], Abid et al.[26] and Rajan et al.[27]. So lyophilized flavedo extract was selected for further studies on the basis of comparatively higher amount of phenolics and flavonoids.
Colour properties (L, a, b values) and various anti- oxidant properties of lyophilized extract powder obtained after extraction are presented in Table 3. The data of color values (L*, a*, b*) of phenolic extract powder show that highest L* (Lightness) value as 45.88 was observed in T2, whereas, lowest L* value as 43.84 was observed in T1. However, the highest a* (Red to green) value as 45.62 in T2 and lowest a* value as 32.81 was observed in T1. Whereas, highest b* (yellow to blue) value as 76.90 was observed in T2 and lowest as in T1. The data in same Table also shows that highest DPPH anti-oxidant activity, metal chelating activity, FRAP as well reducing power was observed in T1 and lowest in T2. The maximum amount of phenolics and flavonoids recorded in T1 in lyophilized wild pomegranate flavedo extract might be due to the better stability of various antioxidant compounds with low temperature as well as higher initial antioxidants values as compared to other treatments. The increased redox potential of polyphenols, which allows them to serve as reducing agents, hydrogen donors and singlet oxygen quenchers, may explain the higher antioxidant activities dried extract[28]. In earlier, studies it is reported that retention of higher antioxidant properties might be due to higher retention of phenolics[11].
| Parameters | Treatments | |
|---|---|---|
| T1 | T2 | |
| L* | 43.84±0.01a | 45.88±0.02b |
| a* | 32.81±0.04a | 45.62±0.01b |
| b* | 67.20±0.10a | 76.90±0.05b |
| DPPH anti-oxidant activity (%) | 84.00±0.45a | 71.10±0.30b |
| Metal chelating activity (%) | 65.20±0.40a | 49.13±0.70b |
| FRAP (μM Fe2+/100 g) | 610.10±0.90a | 411.31±0.71b |
| Reducing power (Absorbance at 700 nm) | 1.617±0.01a | 1.420±0.10b |
Note: Different superscripts lettersa,b in the same column indicate significant differences (p<0.05). T1: Ethanol and distilled water (50:50) extract after 1 h of extraction by reflux method; T2: Acetone and distilled water (50:50) extract after 1 h of extraction by reflux method; *L (Lightness); *a (Red to green) and *b (Yellow to blue)
Table 3: Colour and Antioxidant Properties of Lyophilized Phenolic Extract Powder of Different Treatments
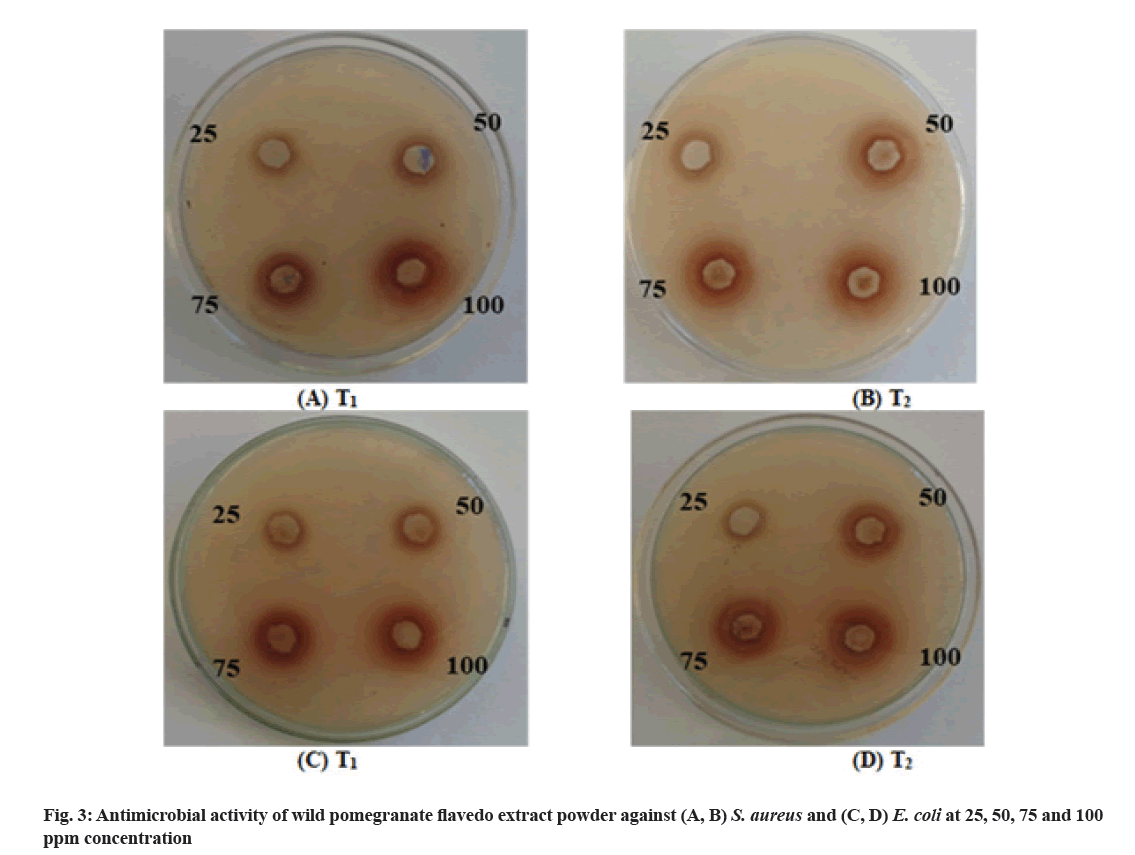
The data presented in Table 4 and fig. 3 show that there was a general increasing trend in inhibition zone (mm) of different lyophilized phenolic extracts powder against S. aureus with increasing the concentration of extract from 25 ppm to 100 ppm. Maximum (21.0 mm) zone of inhibition was found in the treatment T1 at 100 ppm concentration. Whereas, maximum (18.0 mm) zone of inhibition was also found in treatment T1 at 100 ppm concentrations. With the increase in concentration of phenolic extract powder, zone of inhibition against S. aureus and E. coli increased significantly. Similar trend of increasing zone of inhibition has been also reported by Mohamed et al.[29]. The maximum zone of inhibition against S. aureus and E. coli observed in treatment T1 might be due to the presence of higher amount of phenolics and flavonoids in this extract.
| Treatments/Concentrations | S. aureus | E. coli | ||
|---|---|---|---|---|
| Inhibition zone (mm) | Inhibition zone (mm) | |||
| T1 | T2 | T1 | T2 | |
| 25 ppm | 13.0±0.20a | 10.5±0.10b | 12.2±0.12a | 8.4±0.10b |
| 50 ppm | 15.0±0.18a | 12.0±0.15b | 15.0±0.20a | 10.0±0.18b |
| 75 ppm | 17.5±0.10a | 15.1±0.12b | 17.0±0.22a | 13.0±0.15b |
| 100 ppm | 21.0±0.20a | 17.0±0.11b | 18.0±0.14a | 15.0±0.10b |
Note: Different superscripts lettersa,b in the same column indicate significant differences (p<0.05). T1: Ethanol and distilled water (50:50) extract after 1 h of extraction and T2: Acetone and distilled water (50:50) extract after 1 h of extraction
Table 4: Antimicrobial Activity of Different Lyophilized Phenolic Extracts Powder Against S. aureus and E. coli
The other reason might be due to the antimicrobial activities of phenolic compounds involved in multiple modes of action like degradation of cell wall, interaction with the composition and disruption of cytoplasmic membrane[30], damage of membrane protein, interference with membrane integrated enzymes[31], change in fatty acid and phospholipids constituents, impairing of enzymatic mechanisms for energy production and metabolism, alteration of nutrient uptake and electron transport[32], influenced the synthesis of Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA), and destroyed the protein translocation and the function of mitochondrion in eukaryotes[33]. Hamid et al.[34] have also reported varying zone of inhibition against various microorganisms in different extracts of wild pomegranate peel.
Wild pomegranate flavedo powder could be utilized for the extraction of phenolics through various solvents by reflux method. The highest total phenolics, total flavonoids, DPPH free radical scavenging activity, FRAP and metal chelating activity among different extracts of wild pomegranate flavedo were recorded when combination of ethanol and distilled water (in the ratio of 50:50) was used for extraction (1 h). The selected lyophilized extracts have high antimicrobial activity against S. aureus and E. coli. Hence fruit flavedo is potential source of natural antioxidants, which could be used in the development of new functional food and nutraceuticals for prevention of various diseases in future. This research will be helpful in selection of optimal raw materials for the development of new functional and nutritional supplements.
Acknowledgements:
We sincerely acknowledge Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Nauni, Solan, Himachal Pradesh, India as well as UGC, New Delhi for providing us facility and environment to work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Kher R. A note on the physico-chemical characters of the wild pomegranate (Punica protopunica L.). Ann Biol 1999;15(2):231-2.
- Thakur NS, Bhat MM, Rana N, Joshi VK. Standardization of pre-treatments for the preparation of dried arils from wild pomegranate. J Food Sci Technol 2010;47(6):620-5.
[Crossref] [Google scholar] [PubMed]
- Thakur NS, Dhaygude GS, Gupta A. Physico-chemical characteristics of wild pomegranate fruits in different locations of Himachal Pradesh. Int J Farm Sci 2011;1(2):37-44.
- Parmar C, Kaushal MK. Wild fruits of the Sub-Himalayan region. New Delhi: Kalyani Publisher; 1982. p. 148.
- Thakur A, Thakur NS, Hamid, Kumar P. Studies on physico-chemical and antioxidant properties of wild pomegranate fruits in different locations of Himachal Pradesh, India. Int J Curr Microbiol Appl Sci 2018;7(8):2842-50.
- Hamid, Thakur NS, Thakur A. Microencapsulation of wild pomegranate flavedo phenolics by lyophilization: Effect of maltodextrin concentration, structural morphology, functional properties, elemental composition and ingredient for development of functional beverage. LWT Food Sci Technol 2020;133:110077.
- Akhtar S, Ismail T, Fraternale D, Sestili P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem 2015;174:417-25.
[Crossref] [Google scholar] [PubMed]
- Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 2007;109(2):177-206.
[Crossref] [Google scholar] [PubMed]
- Masci A, Coccia A, Lendaro E, Mosca L, Paolicelli P, Cesa S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem 2016;202:59-69.
- Hamid, Thakur NS, Sharma R, Thakur A. Stability of mango drink enriched with micro-encapsulated pomegranate peel extract. Indian J Hortic 2021;78(3):330-7.
- Hamid, Thakur NS, Thakur A, Kumar P. Effect of different drying modes on phenolics and antioxidant potential of different parts of wild pomegranate fruits. Sci Hortic 2020;274:109656.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16(3):144-58.
- Ilahy R, Hdider C, Lenucci MS, Tlili I, Dalessandro G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J Food Compost Anal 2011;24(4):588-95.
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995;28(1):25-30.
- Oktay M, Gülçin ?, Küfrevio?lu Ö?. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci Technol 2003;36(2):263-71.
- Dinis TC, Madeira VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315(1):161-9.
[Crossref] [Google scholar] [PubMed]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239(1):70-6.
- Zieli?ski H, Koz?owska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem 2000;48(6):2008-16.
[Crossref] [Google scholar] [PubMed]
- Liu X, Dong M, Chen X, Jiang M, Lv X, Yan G. Antioxidant activity and phenolics of an endophytic Xylariasp. from Ginkgo biloba. Food Chem 2007;105(2):548-54.
- Sripad G, Prakash V, Rao MS. Extractability of polyphenols of sunflower seed in various solvents. J Biosci 1982;4(2):145-52.
- Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009;14(6):2167-80.
[Crossref] [Google scholar] [PubMed]
- Musa KH, Abdullah A, Jusoh K, Subramaniam V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal Methods 2011;4(1):100-7.
- Hamid, Thakur NS, Sharma R, Thakur A, Kumar P, Gautam S. Phytochemical extraction and quantification from wild pomegranate flavedo powder, their antioxidant and antimicrobial properties. Ann Phytomedicine 2020;9:187-94.
- Berrueta LA, Alonso-Salces RM, Héberger K. Supervised pattern recognition in food analysis. J Chromatogr A 2007;1158(1):196-214.
[Crossref] [Google scholar] [PubMed]
- Azarpazhooh E, Sharayei P, Zomorodi S, Ramaswamy HS. Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food Bioproc Tech 2019;12(2):199-210.
- Abid M, Yaich H, Cheikhrouhou S, Khemakhem I, Bouaziz M, Attia H, et al. Antioxidant properties and phenolic profile characterization by LC-MS/MS of selected Tunisian pomegranate peels. J Food Sci Technol 2017;54(9):2890-901.
- Rajan S, Mahalakshmi S, Deepa VM, Sathya K, Shajitha S, Thirunalasundari T. Antioxidant potentials of Punica granatum fruit rind extracts. Int J Pharm Pharm Sci 2011;3(3):82-8.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 1999;47(10):3954-62.
[Crossref] [Google scholar] [PubMed]
- Mohamed Z, Ridha OM, Eddine LS, Rebiai A. Phenolic content, antioxidant and antibacterial activities of peel extract from Punica granatum L. Res J Chem Environ 2018;22(4):9-15.
- Lambert RJ, Skandamis PN, Coote PJ, Nychas GJ. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 2001;91(3):453-62.
[Crossref] [Google scholar] [PubMed]
- Raccach M. The antimicrobial activity of phenolic antioxidants in foods: A review. J Food Saf 1984;6(3):141-70.
- Taniguchi M, Yano Y, Tada E, Ikenishi K, Oi S, Haraguchi H, et al. Mode of action of polygodial, an antifungal sesquiterpene dialdehyde. Agric Biol Chem 1988;52(6):1409-14.
- Nychas GJ. Natural antimicrobials from plants. In: Gould GW editor. New methods of food preservation. London: Blackie Academic; 1995. p. 58-89.
- Hamid, Thakur NS, Sharma R, Sharma YP, Gupta RK, Rana N, Thakur A. Phenolics from underutilized wild pomegranate fruit flavedo: Extraction, quantification, hierarchical clustering, antibacterial properties, HPLC, SEM analysis and FT-IR characterization. S Afr J Bot 2022;145:85-94.