- *Corresponding Author:
- J. Han
Department of Orthopedic Surgery, Ruikang Hospital Affiliated to Guangxi University of Traditional Chinese Medicine, Nanning, Guangxi 530011,China
E-mail: jham@163.com
| This article was originally published in a special issue, “RecentDevelopments in Biomedical Research and PharmaceuticalSciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “212-219” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Dieda ointment, a Chinese medicine, has been widely used in the clinical treatment of fracture after years of clinical research to prove that it has significant effects on healing injury and relieving pain, reinforcing tendons and setting bones. However, the mechanism of its effects on fracture healing is still unclear. The purpose of this study was to explore the mechanism of Dieda ointment in promoting fracture healing. The materials and methods explored were used to establish a fracture model of Sprague-Dawley rats, which was externally applied with Dieda ointment for 1, 2 and 4 w and imaging examination of the fracture site was performed. Histological examination was also performed. Western blotting and quantitative reverse transcription polymerase chain reaction were used to detect the expression of proteins and its messenger ribonucleic acid. We found that Dieda ointment can effectively accelerate callus formation at tibia fractures in rats and increase the messenger ribonucleic acid and protein expression levels of bone morphogenetic protein-2, suppressor of mothers against decapentaplegic 1, runt-related transcription factor 2 and Sp7 transcription factor, regulate bone formation and promote fracture healing. The study on the mechanism of Dieda ointment promoting fracture healing, not only provides theoretical and experimental basis for the subsequent study of Dieda ointment.
Keywords
Dieda ointment, bone morphogenetic protein-2, suppressor of mothers against decapentaplegic 1, runt-related transcription factor 2, Sp7 transcription factor, fracture healing
In recent years, with the rapid development of social economy, the popularization of transportation and the prominent aging problem, fracture has become a common health problem. The natural healing of a fracture is a long and complex physiological process and most fractures are usually healed through natural healing rather than accelerated healing[1]. Epidemiologically, there are 5.6 million cases of delayed fracture union in the America each year and approximately 100 000 of these patients will develop non-union[2,3]. At present, the development of orthopedic surgery is increasing, coupled with the introduction of high and new technology, modern medicine has made rapid progress in the treatment of fracture. However, nearly 15 % of patients still suffer from delayed union or non-union[4]. In addition, fracture complications due to improper treatment or other external factors are not rare[5]. The average cost of treating a long bone fracture in Western countries is more than $ 10 000[2]. It is characterized by high surgical costs, increased risk of postoperative infection, unclear recovery time and easy to scar, which often cause great economic and psychological burden to patients[6]. Therefore, how to use a relatively economic way to promote fracture recovery, shorten the treatment cycle of patients and achieve early functional exercise has become an urgent problem to be solved in modern orthopedics, but also become the focus of scholars on domestic and overseas.
Traditional Chinese medicine has a long history in the treatment of fracture. During this period, it has accumulated rich clinical experience, which is easier and safer than Western surgical treatment. Assemble Flavone of Rhizoma drynariae (AFRD) is an effective ingredient extracted from the dry rhizome of Rhizoma drynariae. Studies have shown that AFRD can promote the expression of bone graft mineralization and osteogenic proteins during induction, membrane growth and promote Osteoblast (OB) differentiation by activating the Wingless-related integration site (Wnt)/ beta (β)-catenin signaling pathway[7]. Total glycosides may promote the osteogenic differentiation of Adipose Mesenchymal Stem Cells (ADSCs) and reverses the bone loss induced by ovariectomy in rats, which is mainly due to the inhibition of Notch signaling pathway by total glycosides to stimulate OB differentiation and bone formation[8]. Asperosaponin VI is the main active component of Dipsacus asper Wall. Studies have shown that Asperosaponin VI can increase the synthesis of Bone Morphogenetic Protein-2 (BMP-2) and activate the signaling pathways of p38 and Extracellular Signal- Regulated Kinase 1/2 (ERK1/2), induce the mature differentiation of OBs, promote the proliferation and differentiation of primary OBs, so that it promote bone formation. Asperosaponin VI can also promote the differentiation of Human Mesenchymal Stem Cells (HMSCs) into nucleus pulposus cells[9,10]. Studies have found that emodin can increase the secretion of OB gene markers such as alkaline phosphatase and augment the expression of Runt-related transcription factor 2 (Runx2) and Osteocalcin (OC) in rat’s primary OBs, which can also activate the p38-Runx pathway to enhance OB differentiation, accelerate bone formation and promote fracture healing[11].
Dieda ointment is an experiential ointment developed by Ruikang Hospital affiliated to Guangxi University of Traditional Chinese Medicine for the treatment of tumbling injury. After many years of clinical application, it has been proved that it has significant effect on healing injury and relieving pain, reinforcing tendons and setting bones and it is widely used in the treatment of orthopaedic diseases. Preliminary experimental studies have found that Dieda ointment can improve the expression of Transforming Growth Factor β (TGF-β) and BMP-2, and promote fracture healing. However, the specific mechanism of promoting fracture healing needs to be explored[12,13]. Therefore, we hypothesized that Dieda ointment may promote fracture healing through the BMP-2, Suppressor of mothers against decapentaplegic 1 (Smad1), Runx2, Sp7 Transcription Factor (Sp7) signaling pathway. In this study, we first established a rat fracture model and then treated it with Dieda ointment to finally reveal the mechanism of Dieda ointment promoting fracture healing.
Materials and Methods
Animals and medicines:
The 8 w healthy male Sprague-Dawley (SD) rats were supplied by the Laboratory Animal Center; weight: 180 g-220 g. Experimental rats were placed in the environment of 25°±2° temperature, 50 %±5 % humidity and 12 h light and dark cycles and allow them to access to food and water freely. This study was approved by institutional animal welfare and ethical committee of Guangxi University of Chinese medicine with the approval number-DW20220415-091. Dieda Ointment, a Chinese medicine, is made of 13 Chinese herbs, such as Rhizoma drynariae, Semen persicae, safflower, ground beetle, Eucommia ulmoides, Dipsacus asperoides, rhubarb and Gendarussa ventricosa, with lanolin, peppermint oil, camphor and Vaseline. All the medicines used in this experiment were prepared by the preparation room of Ruikang Hospital affiliated to Guangxi University of Traditional Chinese Medicine according to the established pharmaceutical process. Yunnan Baiyao was produced by Baiyao Group Co., Ltd.
Grouping of animals and methods of medication:
The experimental rats were randomly divided into blank group, model group, control group and therapy group. There were 18 rats in each group. Each group was randomly divided into 1 w group, 2 w group and 4 w group according to different observation times. There were 6 rats in each group. The therapy group used 1 g Dieda ointment, the control group used 1 g powder of Yunnan Baiyao (1/5 of the amount) and normal saline to make paste, and the model group only used normal saline, wrapped in gauze and covered the affected parts of the therapy group, control group and model group respectively. The specific groups are as follows:
Blank group: Normal feeding, no intervention.
Model group: Normal feeding and external application of normal saline after modeling.
Control group: After normal feeding, Yunnan Baiyao was applied to the affected area after modeling.
Therapy group: Normal feeding, Dieda ointment was applied to the affected area after modeling.
Fracture model preparation:
Rats were anesthetized by intraperitoneal injection of 1 % sodium pentobarbital (35 mg/kg per SD rat). The anesthetized rats were covered with gauze on their left leg to avoid skin contusion and bleeding during modeling and the rats were protected from other injuries during fracture modeling. A 500 g orthopaedic traction weight was used to drop vertically from the height of 35 cm between the two baffles to smash the middle and lower part of the left calf of rats, resulting in the fracture of the middle and lower part of the tibia of rats. After the fracture model was established, the epidermis and body hair were scraped off and applied externally. The left leg fracture of rats was fixed with a splint around the dressing gauze and a self-locking nylon band was tightened. The band was kept moving up and down about 2 mm to prevent the blood supply of the affected limb. Change the dressing every 2 d according to the above method, in case the medicine becomes dry and affects the curative effect. 80 000 units of penicillin were injected for 3 consecutive days to prevent infection.
Sampling methods:
Digital X-ray or Digital Radiography (DR) examination was performed on the tibia of rats after the 1, 2 and 4 w of treatment. Then all the rats were euthanized and the left tibia was completely dissected, stripping the surrounding skeletal muscle and soft tissue. The tibia of rats were examined histologically and detected by Western blotting technique and quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR).
Hematoxylin-Eosin (HE) staining:
The tibia was immobilized in 4 % paraformaldehyde Phosphate Buffered Saline (PBS) solution for 3 d and then the fixed bone tissue was placed in Ethylenediamine Tetraacetic Acid (EDTA) decalcification buffer for 4 w. The decalcified tibia tissue was embedded in paraffin and cut into 5 μm sections. The paraffin sections were dewaxed with water successively and then the sections were dyed in hematoxylin and eosin dyes. Finally, the sections were dehydrated and sealed for microscopic examination, and then the images were collected and analyzed.
qRT-PCR detection:
Tibial tissue was ground in liquid nitrogen and total Ribonucleic Acid (RNA) was extracted using TRIzol reagent (Invitrogen, Carlsbad, California). The complementary DNA (cDNA) was synthesized using a cDNA synthesis kit (Takara, Dalian, China). Then, the expression level of each messenger RNA (mRNA) was detected by qRT-PCR and the relative expression level of each mRNA was calculated by 2-ΔΔCt method. Primer sequences are shown in Table 1.
| Name | Primer sequence | Product length (base pair (bp)) |
|---|---|---|
| BMP-2 | Forward: GACCCGCTGTCTTCTAGTGT | 150 |
| BMP-2 | Reverse: AACTCAAACTCGCTGAGGACG | |
| Smad1 | Forward: TCAATAGAGGAGATGTTCAAGCAGT | 134 |
| Smad1 | Reverse: GAAACCATCCACCAACACGC | |
| Runx2 | Forward: CGCCTCACAAACAACCACAG | 130 |
| Runx2 | Reverse: AATGACTCGGTTGGTCTCGG | |
| Sp7 | Forward: GCCAGTAATCTTCGTGCCAGAC | 78 |
| Sp7 | Reverse: ATAGTGAGCTTCTTCCTGGGGA | |
| GAPDH | Forward: TCTCTGCTCCTCCCTGTTCT | 91 |
| GAPDH | Reverse: GTTCACACCGACCTTCACCA |
Table 1: Primers for qRT-PCR Analysis
Western blot detection:
The tibial tissue was ground in liquid nitrogen, which is dissolved with a solution buffer. Protein concentration was determined using Bicinchoninic Acid (BCA) method (Solarbio, R0010). The samples were separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel (Servicebio, G2003) and transferred to Polyvinylidene Difluoride (PVDF) membrane by electrophoresis. After being sealed in 5 % skim milk solution for 1 h, the membrane was coated with BMP-2 alpha (α) antibody (Solarbio, K000320P), Smad1 antibody (Bioss, BS-16376R), Runx2 antibody (Bioss, BS-1134R), Sp7 antibody (Solarbio, K009919P) and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) (Solarbio, K106389P) antibody, which were incubated at 37° for 2 h. Wash on Tris Buffered Saline with Tween® 20 (TBST) decolorizing shaker for 3 times at room temperature for 15 min each. Secondary antibody incubation was continued for 1.5 h as described above. It was treated with Enhanced Chemiluminescence (ECL) solution (Biosharp, BL523A). Gel imaging was used to photograph the film and the gray value of each protein band was measured. The gray ratio of target protein to GAPDH in each channel was analyzed to calculate the relative protein expression level.
Data analysis:
The experimental data were analyzed and processed by Statistical Package for the Social Sciences (SPSS) 25.0 software. All measurement data were expressed in the form of x̄ ±s. p<0.05 and p<0.01 were considered statistically significant while using one-way Analysis of Variance (ANOVA), then a bar chart was drawn using Graphpad Prism 9.0.
Results and Discussion
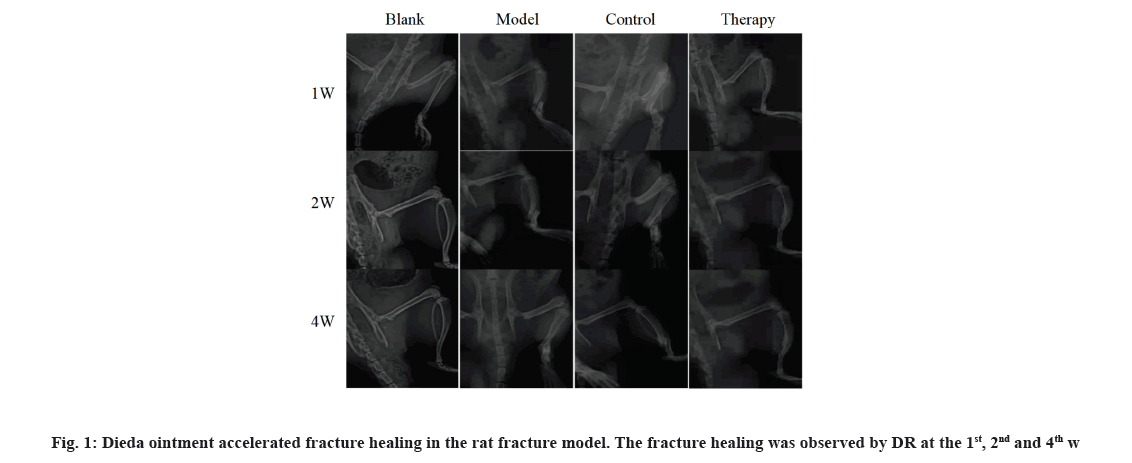
Dieda ointment, a Chinese medicine, can promote fracture healing. The DR images of the tibia of rats showed incomplete healing during the healing process of the 1, 2 and 4 w of topical treatment. Compared with the natural healing of rats in the model group, the callus growth rate of rats in the therapy group treated with Dieda ointment and the control group treated with Yunnan Baiyao was faster and the fracture healing degree was better at the 2nd and 4th w of treatment. DR images showed that Dieda ointment could accelerate fracture healing in rat’s fracture (fig. 1).
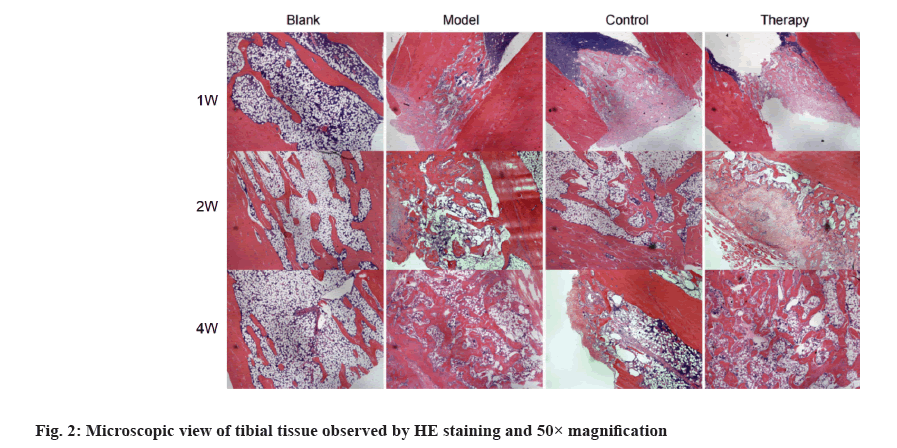
HE staining of tibial bone tissue at 50× magnification, showed that the bone tissue structure of rats in the blank group was normal. In the model group, only a few capillaries were observed and the trabeculae at the fracture end were slender and sparse, with more disordered arrangement, and the spacing between trabeculae became larger and more voids. After the 1st, 2nd and 4th w of external application, compared with the model group, the hematoma in the therapy group and the control group had a faster rate of mechanization. The trabeculae at the fracture were more mature. Many osseous bridge and mineralized bone were formed (fig. 2).
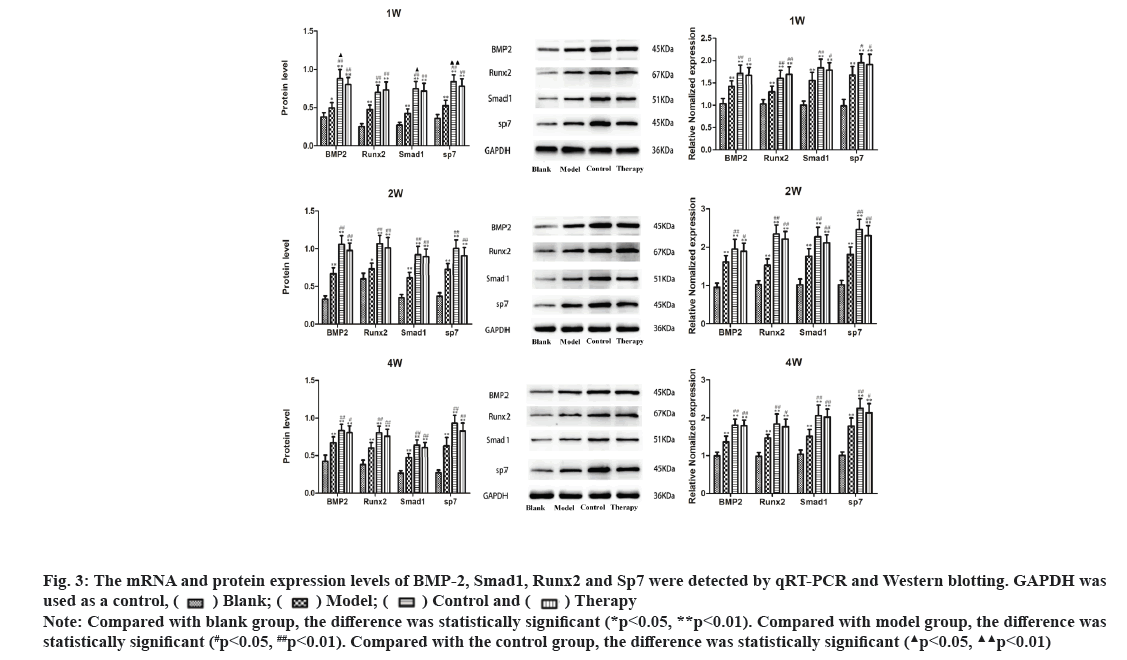
Dieda ointment activates BMP-2, Smad1, Runx2 and Sp7 signaling pathway in rats fracture model. qRT-PCR analysis was used to detect the expression levels of BMP-2, Smad1, Runx2 and SP7 mRNA in the pragmatic model of rats. The results showed that compared with the blank group, the expression of BMP-2 mRNA, Smad1 mRNA, Runx2 mRNA and Sp7 mRNA were significantly increased at the 1st, 2nd and 4th w of treatment (p<0.01). The expression levels of BMP-2 mRNA, Smad1 mRNA, Runx2 mRNA and Sp7 mRNA in therapy group, control group and model group were increased at the 1st, 2nd and 4th w of treatment, and the differences were statistically significant (p<0.05). There were no significant differences in BMP-2 mRNA, Smad1 mRNA, Runx2 mRNA and Sp7 mRNA between the therapy group and the control group at the 1st, 2nd and 4th w of treatment (p>0.05). Western blotting analysis was used to detect the protein expression levels of BMP-2, Smad1, Runx2 and Sp7 in rat fracture models. The results showed that compared with blank group, the expression of BMP-2 protein, Smad1 protein, Runx2 protein and Sp7 protein in model group were increased at the 1st, 2nd and 4th w of treatment and which has statistical significance (p<0.05). The expression levels of BMP-2 protein, Smad1 protein, Runx2 protein and Sp7 protein in therapy group, control group and model group were increased at the 11st, 2nd and 4th w of treatment, with statistical significance (p<0.05). When compared the therapy group with the control group, the expressions of BMP-2 protein, Smad1 protein and Sp7 protein in the 1st w of treatment were increased with statistical significance (p<0.05), but there was no statistical significance in the 2nd and 4th w of treatment (p>0.05). These data suggest that Dieda ointment can activate BMP-2, Smad1, Runx2 and Sp7 signaling pathway (fig. 3).
Figure 3: The mRNA and protein expression levels of BMP-2, Smad1, Runx2 and Sp7 were detected by qRT-PCR and Western blotting. GAPDH was Note: Compared with blank group, the difference was statistically significant (*p<0.05, **p<0.01). Compared with model group, the difference was statistically significant (#p<0.05, ##p<0.01). Compared with the control group, the difference was statistically significant
Note: Compared with blank group, the difference was statistically significant (*p<0.05, **p<0.01). Compared with model group, the difference was statistically significant (#p<0.05, ##p<0.01). Compared with the control group, the difference was statistically significant 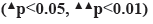
In recent years, more and more studies have explored the effects of traditional Chinese medicine on osteogenesis and fracture healing[14,15]. External application of traditional Chinese medicine has high safety and few adverse reactions. At the same time, it is cheap, easy to use and the medicine effect is remarkable. Dieda ointment, a traditional Chinese medicine, has been widely used in clinical fracture treatment because it contains many active ingredients to promote fracture healing[16,17]. Yunnan Baiyao has been shown to promote fracture healing in studies. It has been widely used in clinical practice for fracture treatment[18]. Therefore, we chose it as a positive control to evaluate the efficacy of Dieda ointment on fracture injury. According to the preliminary experiment, our team selected Dieda ointment for the external application for 1, 2 and 4 w in the rat fracture model and studied the mechanism of Dieda ointment in promoting fracture healing.
In previous experimental studies, it was found that Dieda ointment, a traditional Chinese medicine, can increase the expression of TGF-β1 and BMP-2[12,13]. The BMP- 2/Smad1/Runx2/Sp7 signaling pathway is one of the important regulatory pathways in bone metabolism. The rapid repair mechanism for fractures begins with osteoprogenitor cells. Hematopoietic cells are produced at the site of the fracture, which secrete growth factors.
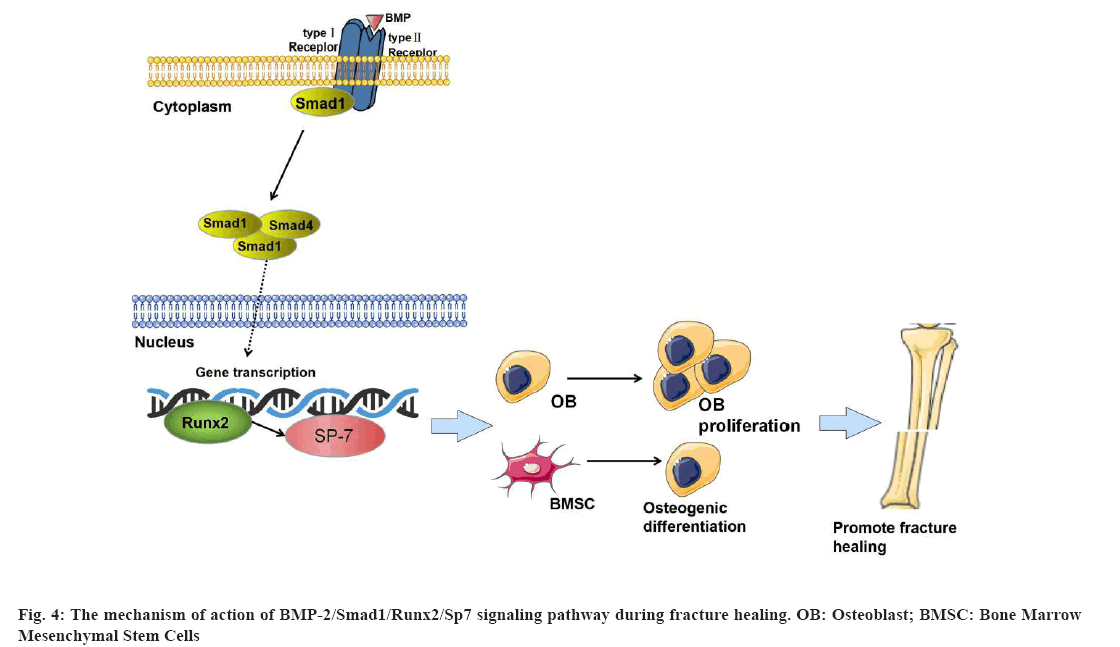
Undifferentiated mesenchymal progenitor cells proliferate and differentiate into chondrocytes and OBs under the co-regulation of BMPs and growth factors, which begin to form bone tissue that can repair bone damage. BMPs are usually divided into at least four subtypes, BMP- 2/4, BMP-5/6/7/8A/8B, BMP-9/10 and BMP-12/13/14, among which BMP-2/4/7 has better osteogenic bioactivity and is closely related to osteogenic differentiation[19]. BMP-2 can stimulate OB differentiation, including the expression of bone structural proteins such as type I collagen and OC, and promote the mineralization of bone matrix[20]. The BMP-2 ligand has two receptors, type I and type II. BMP-2, in turn, binds to specific receptors, Bone Morphogenetic Protein Receptor Type I (BMPR-I) and BMPR Type II (BMPR-II) on the cell membrane which in turn phosphorylate BMPR-I, then binds to downstream Smad1 and phosphorylates Smad1. Phosphorylated Smad1 binds to Smad4 in the cytoplasm to form a complex that is further activated. It is transferred into the nucleus to bind to target genes and can up-regulate Runx2 and Sp7[21]. Runx2 is considered to be a common downstream factor of BMP signaling. Runx2 interacts with Smad1, also a downstream factor of BMP signal transduction to stimulate Bone Marrow Mesenchymal Stem Cells (BMSCs) and regulate OB differentiation[22] which has been confirmed in a large number of in vitro and in vivo experiments. Runx2 is a specific bone transcription factor required for bone development, which acts on OB mediated bone formation and osteoclast mediated bone resorption and participates in bone remodeling at the fracture end. It plays an irreplaceable role in fracture healing[23]. Sp7, also known as Osterix, is an important transcription factor downstream of Runx2. Overexpression of Runx2 in mouse limb bud cells was analyzed by microarray method. Data from the experiment also fully confirmed that Sp7 regulates osteogenic differentiation at the downstream regulatory sites of Runx2 and the expression ability of Runx2 and Sp7 in cells is a positive feedback effect. The expression of Sp7 is regulated by its upstream Runx2, which is related to the eventual maturation of OBs[24]. Runx2 and Sp7 are considered to be central to the regulation of osteogenesis. As a downstream molecule of the BMP-2, Smad1 signaling pathway plays a role in promoting OB proliferation and differentiation. We found that Dieda ointment can promote the formation of callus at the fracture end and accelerate the time of fracture healing. The mRNA and protein expression levels of BMP-2, Smad1, Runx2 and Sp7 were significantly up-regulated after the application of Dieda ointment which is based on the role of BMP-2, Smad1, Runx2, Sp7 signaling pathway in bone metabolism. We found that the promoting effect of Dieda ointment on fracture healing was related to the BMP- 2, Smad1, Runx2, Sp7 signaling pathway. Our results suggest that Dieda ointment regulates bone formation and fracture healing through the BMP-2, Smad1, Runx2, Sp7 signaling pathway (fig. 4).
In this study, Dieda ointment can promote fracture healing in rats by activating the BMP-2, Smad1, Runx2, Sp7 signaling pathway. At present, the study of Dieda ointment promoting fracture healing is still in the initial stage. In the future, our team will study the regulatory relationship between Dieda ointment and BMP-2, Smad1, Runx2, Sp7 signaling pathway, and further explore other related molecular mechanisms.
Author’s contributions:
Yukun Wu should be considered as first author. Jie Han conceived and designed the study. Yukun Wu performed experiments and drafted the manuscript. Shuaibo Wen analyzed data. Guowu Ren and Yuzhi Shang interpreted results of experiments and Zhiwei Xu prepared figures.
Acknowledgements:
This research was supported by the scientific research project funded by administration of Traditional Chinese Medicine of Autonomous Region (GZZC2019084) and Preparation Quality Improvement Project in Guangxi Zhuang Medicine Hospital (GZZJ202011) and Guangxi Zhuang Autonomous Region Youth Qi-Huang Scholar Training Program (Guizhong Medical Science and Education Development [2022] No. 13) and 2022 Central subsidy Guangxi medical services and security capacity improvement (Traditional Chinese Medicine inheritance writing development part); A Chinese doctor master and national famous traditional Chinese medicine inheritance studio, Wealth club (2022).
Conflict of interests:
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: Current concepts and future directions. BMC Med 2011;9(1):1-10.
- Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, et al. Delayed union and nonunions: Epidemiology, clinical issues and financial aspects. Injury 2014;45(2):S3-7.
[Crossref] [Google scholar] [PubMed]
- Haas NP. Callus modulation-fiction or reality? Chirurg 2000,71(9):987-8.
[Crossref] [Google scholar] [PubMed]
- Westgeest J, Weber D, Dulai SK, Bergman JW, Buckley R, Beaupre LA. Factors associated with development of nonunion or delayed healing after an open long bone fracture: A prospective cohort study of 736 subjects. J Orthop Trauma 2016;30(3):149-55.
[Crossref] [Google scholar] [PubMed]
- Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury 2007;38(2):S3-9.
[Crossref] [Google scholar] [PubMed]
- Zeckey C, Mommsen P, Andruszkow H, Macke C, Frink M, Stübig T, et al. The aseptic femoral and tibial shaft non-union in healthy patients–An analysis of the health-related quality of life and the socioeconomic outcome. Open Orthop J 2011;5:193-7.
[Crossref] [Google scholar] [PubMed]
- Li S, Zhou H, Hu C, Yang J, Ye J, Zhou Y, et al. Total flavonoids of Rhizoma drynariae promotes differentiation of osteoblasts and growth of bone graft in induced membrane partly by activating Wnt/β-catenin signaling pathway. Front Pharmacol 2021;12:675470.
[Crossref] [Google scholar] [PubMed]
- Zhou YH, Xie Q. Total glycosides from Eucommia ulmoides seed promoted osteogenic differentiation of adipose-derived mesenchymal stem cells and bone formation in ovariectomized rats through regulating Notch signaling pathway. J Orthop Surg Res 2021;16(1):1-10.
[Crossref] [Google scholar] [PubMed]
- Niu Y, Li Y, Huang H, Kong X, Zhang R, Liu L, et al. Asperosaponin VI, a saponin component from Dipsacus asper Wall., induces osteoblast differentiation through bone morphogenetic protein‐2/p38 and extracellular signal‐regulated kinase 1/2 pathway. Phytother Res 2011;25(11):1700-6.
[Crossref] [Google scholar] [PubMed]
- Niu YT, Xie L, Deng RR, Zhang XY. In the presence of TGF-β1, Asperosaponin VI promotes human mesenchymal stem cell differentiation into nucleus pulposus like-cells. BMC Complement Med Ther 2021;21(1):1-11.
[Crossref] [Google scholar] [PubMed]
- Kim JY, Cheon YH, Kwak SC, Baek JM, Yoon KH, Lee MS, et al. Emodin regulates bone remodeling by inhibiting osteoclastogenesis and stimulating osteoblast formation. J Bone Miner Res 2014;29(7):1541-53.
[Crossref] [Google scholar] [PubMed]
- Han J, Wang DW, Mo J, Chen Y. TGF-β1 expression mechanism of traditional Chinese medicine on fracture healing. Jilin J Tradit Chin Med 2016;36(5):494-6.
- Han J, Wang DW, Mo J, SU Bo, Chen Y. Applied basic research on effect of Chinese medicine Dieda ointment on TGF-β1 expression in fracture healing. China Foreign Medical Treat 2016;35(15):158-60.
- Zhou MT, Feng L, Tao KJ, Zheng JP, Huang JB, Lin HY. Effect of traditional Chinese medicine on osseointegration and bone absorption of implants. Zhonghua Kou Qiang Yi Xue Za Zhi 2018;53(10):716-20.
[Crossref] [Google scholar] [PubMed]
- Mukwaya E, Xu F, Wong MS, Zhang Y. Chinese herbal medicine for bone health. Pharm Biol 2014;52(9):1223-8.
[Crossref] [Google scholar] [PubMed]
- Feng Z, Lian KJ, Lin ZH, Chen YP, Cheng Q. Manipulative reduction combined with splint fixation and external Dieda ointment in the treatment of elderly distal sciatic fractures. Chin J Tradit Med Traumatol Orthop 2013;21(3):25-7.
- Han Jie, Mo Jian, Zhu Jianglong, Zuo Jie, Wang Dawei. Clinical value of Dieda ointment combined with open reduction and internal fixation in the treatment of delayed fracture union. Inn Mong J Tradit Chin Med 2016;35(6):74-5.
- Pang X, Wang Y, Wu J, Zhou Z, Xu T, Jin L, et al. Yunnan baiyao conditioned medium promotes the odonto/osteogenic capacity of stem cells from apical papilla via nuclear factor kappa B signaling pathway. Biomed Res Int 2019;2019:1-11.
- Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: A critical review. Cell Signal 2011;23(4):609-20.
[Crossref] [Google scholar] [PubMed]
- Song C, Song C, Tong F. Autophagy induction is a survival response against oxidative stress in bone marrow-derived mesenchymal stromal cells. Cytotherapy 2014;16(10):1361-70.
[Crossref] [Google scholar] [PubMed]
- Axelrad TW, Einhorn TA. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev 2009;20(5):481-8.
[Crossref] [Google scholar] [PubMed]
- Kundu M, Javed A, Jeon JP, Horner A, Shum L, Eckhaus M, et al. Cbfβ interacts with Runx2 and has a critical role in bone development. Nat Genet 2002;32(4):639-44.
[Crossref] [Google scholar] [PubMed]
- Komori T. Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab 2003;21(4):193-7.
[Crossref] [Google scholar] [PubMed]
- Nishimura R, Wakabayashi M, Hata K, Matsubara T, Honma S, Wakisaka S, et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J Biol Chem 2012;287(40):33179-90.
[Crossref] [Google scholar] [PubMed]