- *Corresponding Author:
- L. Wu
Department of Anesthesiology, Xuzhou Municipal Hospital Affiliated with Xuzhou Medical University, China
E-mail: xutie889@163.com
| This article was originally published in a special issue, |
| "Clinical and Experimental Studies on Drug and Intervention Repurposing in China" |
| Indian J Pharm Sci 2019:81(4)spl issue1;175-180 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The choice of premedication is vital in pediatric anesthesia. The aim of this study was to assess the effect of dexmedetomidine on the perioperative period of craniocerebral operation in children. Studies were identified by searching mainstream databases from August 2013 to August 2016 .The assessed outcomes included heart rate, mean arterial pressure, NSE, S100β protein, AV-DO2 and CO2ER. The Cochrane risk biased evaluation tool was used to evaluate the quality of the included literature, and the relevant valid data were extracted. Meta analysis was performed using RevMan 5.3 software. Nine randomized controlled trials and observational studies involving 372 patients were included. The results indicated that children with craniocerebral surgery perioperative who were applicated with dexmedetomidine, the heart rate decreased significantly than the control group (weighted mean difference= -22.72, 95 % CI confidence intervals (-39.2, -6.19), p=0.007), NSE were also decreased (weighted mean differences= -5.14, 95 % confidence intervals (-8.24 -2.03), p=0.001). Moreover, there were significant differences in terms of S100β and mean arterial pressure as well. Current evidence indicates that dexmedetomidine can increase cerebral hemodynamic stability, reduce the cerebral oxygen metabolism rate, decrease NSE and S100 protein in children undergoing craniocerebral surgery. Dexmedetomidine played a significant role in brain protection, which was ideal choice of adjuvant treatment in the perioperative period of craniocerebral operation in children.
Keywords
Dexmedetomidine, craniocerebral, children, meta-analysis
When craniocerebral trauma, aneurysm and brain tumour occurred in children, it is necessary to perform craniotomy under the state of general anaesthesia. However, the stimulation of craniocerebral operation itself can lead to surrounding normal brain tissue mechanical or ischemic injury caused by different influences on the prognosis[1]. Mortality rates of children with craniocerebral trauma are very high. During the perioperative period of craniocerebral operation, the stability of hemodynamics and brain oxygen metabolism are essential for improving the long-term quality of life in children[2]. As the paediatric patients are a special group, development of the brain tissue and systemic organs were not mature so that they are especially susceptible to the influence of narcotic drugs, which is more likely to aggravate nerve damage[3]. So, in recent years, doctors are focused on how to effectively protect brain tissue during craniocerebral surgery. The appropriate use of the perioperative period anaesthetic drugs could effectively protect brain tissue and improved of prognosis.
Dexmedetomidine (DEX), a highly selective α-2 adrenergic receptor agonist, plays a major role in the central nervous system locus coeruleus. It exerts sedative and analgesic effect without affecting intracranial pressure and respiration[4]. DEX protects the stability of brain hemodynamic by reducing brain extracellular catecholamine level and regulating cell apoptosis. Several studies have reported that DEX reduced hypertension and tachycardia in patients undergoing neurosurgery. There are less research currently in clinical about the effects of DEX on paediatric neurosurgical patients. Further studies are required to determine the safety and efficacy of using DEX in children with craniocerebral surgery. The purpose of this study was to evaluate the effects of DEX on paediatric hemodynamics in patients with craniocerebral surgery by meta-analysis. The study protocol followed the proposals of the PRISMA statement and Cochrane Collaboration for systematic reviews and meta-analysis.
PubMed, Cochrane Library, Medicine, Web of Science, Chinese Journal Full-text Database (CNKI), Wanfang Database and other biomedical databases were searched, using a combination of medical subject headings and free words to perform basic searches, using the following keywords, DEX, children, child, paediatric, adolescent, supratentorial tumour, craniotomy, cerebral aneurysm, brain, operation, intracranial, epileptic focus and craniocerebral. Bibliographic reference lists of studies selected for inclusion in our meta-analysis and relevant review articles were manually searched.
All literature has no language or publication date restrictions and the results were collected and duplicate data deleted at EndNote X7. The inclusion criteria for articles selected for meta-analysis were the following, randomized controlled trial design; the original study compared the effects of DEX intravenous administration and placebo assisted anaesthesia in elective surgery for brain surgery in juvenile patients; provide at least one of the following outcome measures, arteriovenous oxygen content difference (AVDO2), cerebral extraction of oxygen (CO2ER), mean arterial pressure (MAP), heart rate (HR), NSE protein, S100β protein and availability of full text. Data from scientific meetings, correspondence, case reports, reviews, and animal studies were excluded.
When there are two or more outcomes, statistical analysis uses Review Manager (version 5.3, The Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark). The continuous variables (HR, MAP) were analysed using weighted mean differences (WMD) or standardized mean differences. When the p value <0.05, the difference was statistically significant. We compared relative ratios for WMD for continuous data with corresponding 95 % confidence intervals (95 % CI) for each trial. I2 test was used to evaluate the heterogeneity of the study. For low heterogeneity result data (I2<30 %), a fixed effect model was used for analysis. For heterogeneous outcome data (I2>30 %), sensitivity analysis results using random effects models were used.
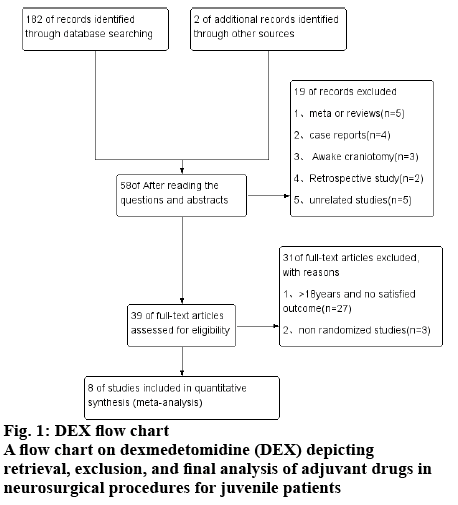
There are 184 articles obtained through the use of theme words and free word retrieval methods, 126 titles or abstracts not relevant to the study question for inclusion and remained 58. Reading the full article included 8 randomized controlled trials, the meta analysis involving a total of 372 cases of juvenile patients, the specific process is shown in fig. 1.
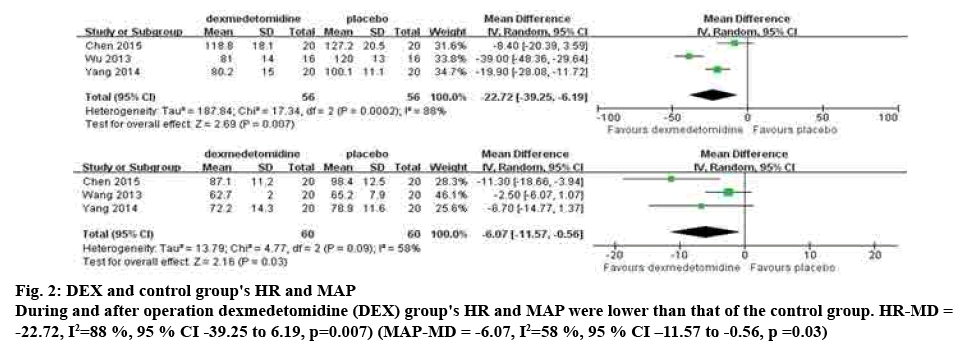
Methodological quality assessment of the 8 RCT articles included were shown in Table 1. All trials were randomized and double-blinded. There were 4 studies with randomized methods and 2 studies detailed the double-blinded approach. None of the studies clearly reported how the allocation was hidden. In 3 studies (n=112) the investigators compared the extubation HR in the DEX group and placebo group. There was strong evidence that HR was lower in the DEX group (WMD=-22.72, 95 % CI [-39.2, -6.19], p=0.007). Heterogeneity among the studies (p= 0.0002, I2=88 %) was heterogeneous among the studies, and used the random effects model.
| Study | A | B | C | D | E | F | Total |
|---|---|---|---|---|---|---|---|
| Wu 2015 | + | ? | + | ? | + | + | 4 |
| Yang 2106 | + | ? | - | ? | + | - | 2 |
| Yao 2016 | + | ? | + | ? | + | - | 3 |
| Yang 2014 | + | ? | - | ? | + | - | 2 |
| Jiang 2016 | + | ? | + | ? | + | - | 3 |
| Chen 2015 | + | ? | + | ? | + | + | 4 |
| Wu 2013 | + | ? | + | ? | + | - | 3 |
| Wang 2013 | + | ? | - | ? | + | - | 2 |
A: sequence generation; B: allocation concealment; C: blinding of participants, personnel and outcome assessors; D: incomplete outcome data; E: no selective outcome reporting; F: other sources of bias +: yes; -: no; ?: unclear
Table 1: Risk of bias assessment for evaluation the quality of each included trials
Three studies (n=120), meta-analysis showed that the MAP was lower in children receiving DEX than placebo (WMD=-6.07, 95 % CI [-11.57, -0.56, p=0.03). The study of heterogeneity test (p= 0.09, I2=58 %), the study of heterogeneity is great, analysis of random effect model, specifically in fig. 2.
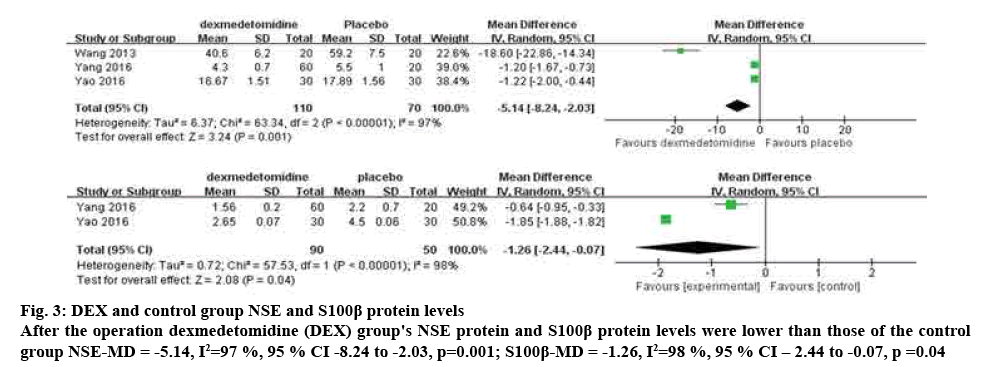
Study on the change of NSE protein between DEX group and placebo group were compared during the postoperative time. Meta-analysis showed that the NSE protein decreased significantly after the use of DEX (WMD=-5.14, 95 % CI [-8.24, -2.03], p=0.001). Heterogeneity among tests (p<0.00001, I2=97 %) was selected by random effect model analysis. Two studies (n=140), the researchers compared the change of S100 protein in the DEX group and placebo group[5,6]. When the data were pooled, DEX group was found to significantly reduce the of S100 protein (WMD=-1.26, 95 % CI [-2.44, -0.07], p=0.04). Heterogeneity among studies (p<0.00001, I2=98 %) was selected by random effect model analysis, as shown in fig. 3.
Figure 3: DEX and control group NSE and S100β protein levels
After the operation dexmedetomidine (DEX) group's NSE protein and S100β protein levels were lower than those of the control group NSE-MD = -5.14, I2=97 %, 95 % CI -8.24 to -2.03, p=0.001; S100β-MD = -1.26, I2=98 %, 95 % CI – 2.44 to -0.07, p =0.04
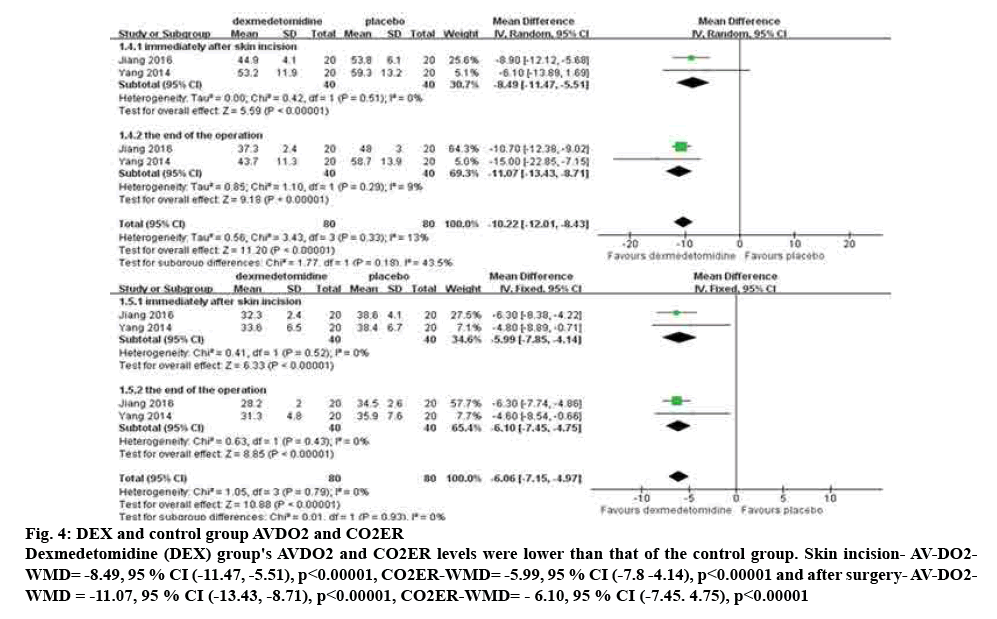
Compared the skin incision and the AV-DO2 and CO2ER changes between DEX group and placebo group[7,8]. The results of meta-analysis are shown that AVDO2 and CO2ER was lower in children receiving DEX than placebo (skin incision (AV-DO2-WMD=-8.49, 95 % CI [-11.47, -5.51], p<0.00001), (CO2ER-WMD=-5.99, 95 % CI [-7.8 -4.14], p<0.00001), after surgery (AV-DO2-WMD=-11.07, 95 % CI [-13.43, -8.71], p<0.00001), (CO2ER-WMD=-6.10, 95 % CI [-7.45, 4.75], p<0.00001). The study of heterogeneity test (p=0.29, I2=9 %), the study of heterogeneity is great, analysis of random effect model, specifically in fig. 4.
Figure 4: DEX and control group AVDO2 and CO2ER
Dexmedetomidine (DEX) group's AVDO2 and CO2ER levels were lower than that of the control group. Skin incision- AV-DO2-WMD= -8.49, 95 % CI (-11.47, -5.51), p<0.00001, CO2ER-WMD= -5.99, 95 % CI (-7.8 -4.14), p<0.00001 and after surgery- AV-DO2-WMD = -11.07, 95 % CI (-13.43, -8.71), p<0.00001, CO2ER-WMD= - 6.10, 95 % CI (-7.45. 4.75), p<0.00001
This systematic review and meta-analysis indicate that the use of DEX is associated with better outcomes in paediatric patients undergoing the perioperative period of craniocerebral operation. The study showed that the HR of was significantly reduced in the children with DEX. The average arterial pressure in the DEX group was reduced and the dosages of other anaesthetics, such as fentanyl and sevoflurane, were reduced as well during the operation, which is consistent with another recent meta-analysis[9]. In addition, the use of DEX didn’t increase the extubation time and perioperative bradycardia risk. Consequently, the patient's hemodynamic stability is maintained.
During craniocerebral operation, the skull-pin headholder was must used to stabilize the head. But that operation was an intense nociceptive stimulus, which could increase blood pressure and cerebral blood flow under general anaesthesia. These brain hemodynamic responses might cause brain oedema, intracranial hypertension, or intracranial haemorrhage in the process of operation. This stress response is characterized by an increase in the secretion of pituitary hormones and the activation of the sympathetic nervous system. It is mainly associated with the increasing of the release of catecholamine and cortisol.
Because of young age, light weight, general complications of malnutrition at different degrees, immaturity of organs and poor compensation abilities, the operative anaesthesia of infants is special. The brain development of children is immature and sensitive to the anaesthetic, which can easily destroy the stability of hemodynamics, appeared respiratory depression, delirium and other side effects. So, the rational use of anaesthetics is particularly significant.
DEX is essentially a selective α-2 adrenergic receptor agonist that binds the receptor with an affinity that is 7~8 times greater than clonidine, a similar drug. The binding of DEX to the receptors reduces the release of intracranial catecholamine, but also can inhibit the activity of the sympathetic nervous system. Then the release of catecholamine neurotransmitter and cortisol are reduced[10]. There also some studies that suggested that DEX can increase the level of calcium in brain blood vessels and reduce glutamate excitotoxicity and the apoptosis of nerve cells. Because of the above reasons, DEX can improve the blood flow, reduce release of inhibited neurons in brain ischemia area and maintain hemodynamic stability. Thus, DEX achieve the protective effect of brain tissue[11].
In the present analysis, the NSE and S100β proteins were significantly lower in the DEX group than in the placebo group at the end of surgery. S100β is a small calcium binding protein. Its biological distribution is extensive and the content of human brain tissue is the highest[12]. NSE is a key enzyme in the glycolytic isozymes of enolase, NSE has 5 isozymes, composed of alpha, beta, gamma subunits to form 2 dimers, including gamma subunits NSE, specifically in normal neurons and neuroendocrine cells[13]. Because the integrity of the blood-brain barrier causes the protein to rarely escape from the brain tissue into the circulatory blood, it is sometimes difficult to extract the protein from the serum[14]. When the ischemic cerebral haemorrhage, traumatic cerebral vascular diseases, such as case, nerve demyelination or neuronal disintegration and destruction of the blood-brain barrier, further leads to a large number of brain neurons of NSE and S100β protein released into the blood circulation[15]. NSE and S100β protein levels were significantly lower in children with brain surgery after DEX assisted anaesthesia than those in the placebo group, suggesting that DEX reduces the release of NSE and S100β proteins.
During the craniocerebral operation, the AV-DO2 and CO2ER in the DEX group were significantly lower than those in the placebo group. The relative cerebral blood flow than the cerebral oxygen metabolism of lower demand to ensure oxygen supply to the brain in the normal range. It suggested that DEX anaesthesia reduced the degree of cerebral anoxia and improved the oxygen metabolism in brain tissue, which achieved the cerebral protective effect[16].
First, several pooled analyses lacked grey literature may lead to publication bias. The quality of the literature included in the study is uneven and there may be bias in implementation and measurement. Second, this meta-analysis was influenced by significant heterogeneity that was introduced by multiple factors that included the difference of DEX dosing time, speed, age and gender. Third, all the intravenous drugs do not collect other medication articles, so different methods of anaesthesia on blood pressure fluctuations may also have certain effect. Future research is needed from the following aspects further improvement: expanding the sample size; random distribution, correct concealment and blinding; extended follow-up.
In conclusion, DEX was found to control postoperative HR and maintain MAP, which was associated with maintaining hemodynamic stability. It also reduced NSE and S100β protein production, which was associated with reducing brain damage. Through reducing AV-DO2 and CO2ER DEX could reduce the cerebral oxygen metabolism. The application of DEX in the perioperative period of craniocerebral operation in children is helpful for the protection of brain tissue and improvement of cure rate.
Acknowledgments
This research was carried out without funding.
Conflict of interest
No conflict of interests among the authors.
References
- Olutoye OA, Baker BW, Belfort MA, Olutoye OO. FDA Warning on Anesthesia and Brain Development: Implications for Obstetric and Fetal Surgery. Am J Obstet Gynecol 2018;218(1):98-102.
- Ibrahim M, Mu’azu AL, Idris N, Rabiu MU, Jibir BW, Getso KI. Menace of childhood non-accidental traumatic brain injuries: A single unit report. Afr J Paediatr Surg 2015;12(1):23-8.
- Fitzsimons J, Bonanno LS, Pierce S, Badeaux J. Effectiveness of preoperative intranasal dexmedetomidine, compared with oral midazolam, for the prevention of emergence delirium in the pediatric patient undergoing general anesthesia: a systematic review. JBI Database System Rev Implement Rep 2017;15(7):1934.
- Dahmani S, Rouelle D, Gressens P, Mantz J. Characterization of the postconditioning effect of dexmedetomidine in mouse organotypic hippocampal slice cultures exposed to oxygen and glucose deprivation. Anesthesiology 2010;112(2):373-83.
- Yang T, Qiu YS. Different doses of dexmedetomidine on brain protection in children with brain tumors during perioperative period. J Tradit Complement Med 2016;22(26):3-4.
- Yao JC. Study on the effects of dexmedetomidine on S100 protein and NSE levels in elderly and young patients with brain trauma. J Taishan Med Univ 2016;37(8):851-3.
- Yang YL, Zhang W, Du P. Dexmedetomidine effects on cerebral hemodynamics and cerebral oxygen metabolism in patients with ischemic moyamoya disease. J Hebei Med Univ 2014;35(1):94-6.
- Jiang B, Song Y, Zhao XB. Effects of dexmedetomidine combined with sevoflurane anaesthesia on cerebral oxygen metabolism in children with EDAS ischemic moyamoya disease. J Hebei Med Univ 2016;37(9):1096-9.
- Wu L, Lv H, Luo W, Jin S, Hang Y. Effects of dexmedetomidine on cellular immunity of perioperative period in children with brain neoplasms. Int J Clin Exp Med 2015;8(2):2748-53.
- Akpinar O, Naziroglu M, Akpinar H. Different doses of dexmedetomidine reduce plasma cytokine production, brain oxidative injury, PARP and caspase expression levels but increase liver oxidative toxicity in cerebral ischemia-induced rats. Brain Res Bull 2017;130:1-9.
- Aryan HE, Box KW, Ibrahim D, Desiraju U, Ames CP. Safety and efficacy of dexmedetomidine in neurosurgical patients. Brain Inj 2006;20(8):791-8.
- Benggon M, Chen H, Applegate R, Martin R, Zhang JH. Effect of dexmedetomidine on brain edema and neurological outcomes in surgical brain injury in rats. Anesth Analg 2012;115(1):154-9.
- Constantin JM, Perbet S. Dexmedetomidine: Superiority trials needed? Reply. Anaesth Crit Care Pain Med 2016;35(3):239.
- Degos V, Charpentier TL, Chhor V, Brissaud O, Lebon S, Schwendimann L, et al. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiology 2013;118(5):1123-32.
- Endesfelder S, Makki H, Von Haefen C. Neuroprotective effects of dexmedetomidine against hyperoxia-induced injury in the developing rat brain. PLoS One 2017;12(2):e0171498.
- Flower O, Hellings S. Sedation in traumatic brain injury. Emerg Med Int 2012;2012:637171.