- *Corresponding Author:
- M. R. Namavar
Department of anatomical sciences, School of medicine, Shiraz university of medical sciences, Shiraz - 71348-53185, Iran
E-mail: namavarm@sums.ac.ir
| Date of Submission | 2 May 2006 |
| Date of Revision | 3 March 2007 |
| Date of Acceptance | 7 August 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 582-585 |
Abstract
Cyclosporine A is an immunosuppressive agent, which is widely used for organ transplantation, autoimmune and other diseases. Cyclosporine inhibits the calcineurin and thereby causes inhibition of chondrogenesis. Cyclosporine crosses the placenta and reaches to the embryo, and thereby may affect them. Many females, that should receive cyclosporine, are in childbearing age. The objective of this study was to determine whether cyclosporine administration has any effect on weight and crown-rump length of mouse embryos. Many out bred adult mice were selected. Female mice were mated overnight with males, checked in the morning for vaginal plug. Pregnant mice were randomly assigned into experimental and control groups. The experimental group received cyclosporine and control group received an equivalent amount of olive oil on days 7, 8, and 9 of gestation. Pregnant mice were killed and embryos were removed on days 13, 16, and 18 of gestation. The weight and crown-rump length of embryos were recorded. Comparison between two groups was made using t. test. There were highly significant differences between crown-rump length of experimental and control groups on days 16 and 18 ( P <0.05), but on day 13, crown-rump length difference was not significant. In all ages of gestation, the experimental group had less weight than the control group ( P <0.05). Results of this study indicate that cyclosporine causes low weight and low crown-rump length in mice embryos.
Keywords
Cyclosporine, weight, Crown-rump length, Pregnancy, Mouse embryo
Cyclosporine A (CsA) is a selective and powerful immunosuppressive agent which is widely used for organ transplantation [1-5], autoimmune diseases [6-8], and also for other diseases [9]. CsA inhibits the activation of calcineurin [10,11] and thereby causes inhibition of chondrogenesis [12], and muscle mass change [13]. CsA crosses the placenta blood barrier reaches to the embryo [2,4,14,15]. Many female patients that they should receive CsA, are in childbearing age or pregnant [1,9,16]. No systematic overview of the use of CsA in pregnancy has been conducted so far, and there are few controlled studies on CsA administration during pregnancy [1,2], although one case of CsA-induced osseous malformation, including limb defects, has been reported [17]. Also few studies address the weight or height of embryos. Furthermore, many studies have used a combination of medications and effects of individual agents on pregnancy have not been well established [1,2,4,5,18-20]. Other problems exist exclusively in organ transplant recipients (or other diseases), such as hypertension that may have also same impacts on birth weight and crown-rump length (CRL) [1,21].
The primary objective of this study was to determine whether CsA exposure in uterus is associated with low weight and low CRL of embryos.
All efforts were made to minimize both the suffering and the number of the animal used. In this study, the humane killing of the mice and embryos collection was carried out after approval by the Ethics Committees of Shiraz University of Medical Sciences.
In this experimental study, many out bred adult (male and female) mice were kept in a standard condition in separate cages [22,23] for one month. Female mice (50) were mated overnight with males, checked in the morning for vaginal plugs. The first day of gestation was considered to the day after plug was found [23]. CsA was dissolved in olive oil at concentration of 15 mg/ml [24]. Two groups of pregnant mice were randomly selected and were assigned into experimental and control groups. The experimental group received an intraperitoneal injection of CsA at dosage of 50 mg/ kg/day of body weight [25] and control group received an equivalent amount of olive oil on days 7, 8, and 9 of gestation.
First, the mice were anesthetized with ether inhalation and then killed by cervical dislocation [23] on days 13, 16, and 18 of gestation. The uterus and its contents were exposed by laparatomy. The weight and CRL of embryos were recorded. A comparison between two groups was made using Student’s t test. Comparisons resulting in P<0.05 were considered significant.
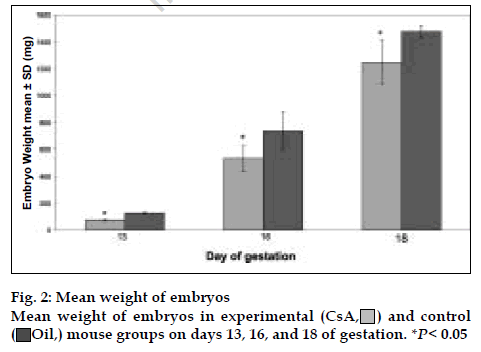
There were 235 embryos in the experimental group and 95 in the control group. The number of embryos decreased in CsA treated group and there were 120 embryo absorption sites in experimental group [25]. In all ages of gestation, the experimental group had less CRL than the control group (fig. 1), but on day 13, there was not significant difference between experimental and control groups. On days 16 and 18, there were highly significant differences between experimental and control groups (P<0.05). The ratio of CRL in experimental to control group was 0.97, 0.89, and 0.85 on days 13, 16, and 18 of gestation, respectively. Therefore, CsA effect on CRL was higher in the last days of pregnancy than earlier days.
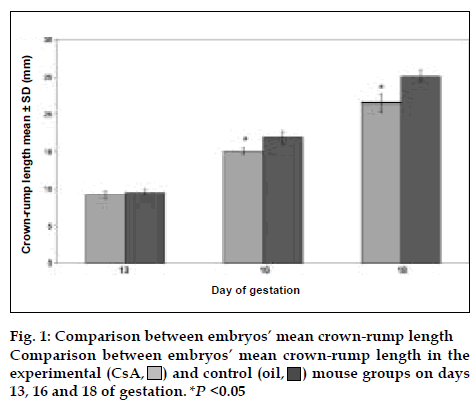
Fig. 2 illustrates the comparison of CsA effect on the weight of embryos. In all ages of gestation, the experimental group embryos had less weight than the control group and there were highly significant differences between experimental and control groups (P <0.05). The ratio of weight in experimental to control group was 0.56, 0.72, and 0.85 on days 13, 16, and 18 of gestation, respectively. Thus, CsA effect on weight was higher in the early days of gestation than last days. As shown in figs. 1 and 2, these results revealed that the mice treated with CsA had a significantly higher frequency of low weight and low CRL than the mice treated with olive oil alone.
According to our data, CsA decreases CRL and weight of embryos. It supports the findings of other studies related to CsA effect on pregnancy outcomes.
There is little research on CsA effect on the height or CRL of embryos. Sgro et al. [19] showed that the embryo’s length was significantly lower in the study group as compared to the control group. Also, Pujals et al. [17] have reported that CsA decreased length of lower limb. In the present study, CsA effect on CRL was not significant in early days of gestation. Also other researchers have shown that in vitro treatment of CsA can interfere with mesenchyme and DNA content and inhibit chondrogenesis on limb bud [26,27] or can induce some osseous malformations [17] and bone loss [20]. CsA, via inhibition of calcineurin, can inhibit chondrogenesis process [12] and skeletal muscles mass change [13]. CsA effects on cartilage and muscles can cause, direct or indirect, shortening of limbs and body, although more researches are necessary to prove this subject.
Several reports have shown that children born by mothers who had taken CsA often present with the low birth weight (LBW), intrauterine growth retardation or small for gestation age [17,24]. Our data accords with other studies on the effect of CsA on weight of embryos. Rahbar and Forghani [28] reported that 77% of newborns, whose mothers used CsA, had LBW. There are similar reports about LBW in infants whose mothers used CsA alone or with combination to other drugs [29]. For example, LBW percentage reported by Lamarque et al. [14] was 44.3%, Armenti et al.21 44-65%, Janssen and Genta7 50%, and Moon et al.30 63.5%. Moon et al.[30] have reported that LBW of neonates was related to their mother’s hypertension. They compared infants of hypertensive and nonhypertensive mothers, although there was LBW in infants whose mothers did not have hypertension but received CsA.
The dosage used in this study considerably exceeds what is normally given to transplant recipients and other patients, although some patients have been given as much as 25 mg/kg/day or also 30 mg/kg/day in rats and 100 mg/kg/day in rabbits [31]. Gasser et al. [24] also used 50 mg/kg/day in mice for teratogenicity. The results of this study indicate that CsA causes low weight and low CRL in mice embryos, as compared with those published in the recent literature with CsA. CsA mostly decreased the weight and CRL of mouse embryos in the earlier and last days of pregnancy, respectively. It is recommended that the drug should not be taken during pregnancy, unless the benefit to the patient outweighs the risk for the fetus. Obviously, more research should be done on the subject.
Acknowledgements
The support provided by the Vice-Chancellor for Research of Shiraz University of Medical Sciences is gratefully acknowledged. We wish to thank the personnel at animal house in Shiraz Medical School.
References
- Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: A meta-analysis. Transplantation 2001;71:1051-5.
- Giudice PL, Dubourg L, Hadj-Aissa A, Saϊd MH, Claris O, Audra P, et al. Renal function of children exposed to cyclosporine in utero. Nephrol Dialysis Transplant 2000;15:1575-9.
- Petri JB, Schurk S, Gebauer S, Haustin UF. Cyclosporine A delays wound healing and apoptosis and suppresses Activin β-A expression in Rats. Eur J Dermatol 1998;8:104-13.
- Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporin, an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation in organ transplantation. Drugs 2001;61:1957-2016.
- Barrou BM, Gruessner AC, Sutherland DE, Gruessner RW. Pregnancy after pancreas transplantation in the cyclosporine era. Transplantation 1998;65:524-7.
- Hern S, Harman K, Bhogal BS, Bhogal BS, Black MM. A Severe persistent case of pemphigoidgestationis treated with intravenous immunoglobulins and cyclosporin. ClinExpDermatol 1998;23:185-8.
- Janssen NM, Genta MS. The effects of immunosuppressive and anti-inflammatory medications on fertility, pregnancy, and lactation. Arch Intern Med 2000;160:610-9.
- Keown PA. Emerging indications for the use of cyclosporine in organ transplantation and autoimmunity. Drugs 1990;40:315-9.
- Imai N, Watanabe R, Fujiwara H, Ito M, Nakamura A. Successful treatment of impetigo herptiformis with oral cyclosporine during pregnancy. Arch Dermatol 2002;138:128-9.
- Ho S, Clipstone N, Timmermann L, Northrop J, Graff I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. ClinImmunolImmunopathol 1996;80:S40-5.
- Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreibe. Calcineurin is a common target of cyclophilin-cyclosporin A and FK506 complexes. Cell 1991;66:807-15.
- Zakany R, Szijgyarto Z, Matta C, Juhasz T, Csortos C, Szucs K, et al. Hydrogen peroxide inhibits formation of cartilage in chicken micromass cultures and decreases the activity of calcineurin: Implication of ERK1/2 and Sox9 pathways. Exp Cell Res 2005;305:190-9.
- Aoki MS, Miyabara EH, Soares AG, Salvini TF, Moriscot AS. Cyclosporin-A does not affect skeletal muscle mass during disuse and recovery. Braz J Med Biol Res 2006;39:243-51.
- Lamarque V, Leleu MF, Monka C, Krupp P. Analysis of 629 pregnancy outcomes in transplant recipients treated with sandimmum. Transplant Proc 1997;29:2480.
- Pervot A, Martini S, Guignard JP. In utero exposure to immunosuppressive drugs. Biol Neonate 2002;81:73-81.
- Vennarecci G, Pisani F, Tison G, Buonomo O, Romagnoly J, Pasqua C, et al. Kidney Transplantation and Pregnancy. Transplant Proc 1997;29:2797-8.
- Pujals JM, Figueras G, Puig JM, Lloveras J, Aubia J, Masramon J. Osseous malformation in baby born to woman on cyclosporine. Lancet 1989;25:667.
- Bakr MA, Ghaneim AES, Fouda MA, Sally S, Moustafa FE, Sobh MA, et al. Clinical course and outcome of pregnancies in recipients of renal allografts. Transplant Proc 1997;29:2787-9.
- Sgro MD, Barozzino T, Mirghani HM, Sermer M, Moscato L, Akoury H, et al . Pregnancy Outcome Post Renal Transplantation. Teratology 2002;65:5-9.
- Fornoni A, Cornacchia F, Howard GA, Roos BA, Striker GE, Striker LJ. Cyclosporine A affects extracellular matrix synthesis and degradation by mouse MC3T3-E1 osteoblasts in vitro .Nephrol Dial Transplant 2001;16:500-5.
- Armenti VT, McGrory CH, Cater JR, Radomski JS, Moritz MJ. Pregnancy outcomes in female renal transplant recipients. Transplant Proc 1998;30:1732-4.
- Little CW, Castillo B, Diloreto DA, Cox C, Wyatt J, Delcerro C. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci 1996;37:204-11.
- Suckow MA, Danneman P, Braytone C. The laboratory mouse. CRC Press: Boca Raton (Florida); 2001. p. 22, 27, 30, 112.
- Gasser DL, Yang P, Buetow KM. Palate teratogenicity and embryotoxicity of cyclosporina in mice. J Craniofac Genet DevBiol 1992;12:155-8.
- Bahmanpour S, Namavar MR. The effects of cyclosporine on pregnancy outcome: The number and mortality of mice embryos. Ahwaz Scientific Medical Journal 2002;33:1-4.
- Bahmanpour S, Paulsen DF. Cyclosporine effects on chick limb bud mesenchyme and DNA content. Indian J Med Sci 2003;28:180-4.
- Bahmanpour S, Paulsen DF. Inhibition of chondrogenic differentiation in chick limb-bud mesenchyme microcultures treated with cyclosporine. Indian J Pharmacol 2006;38:43-8.
- Rahbar K, Forghani F. Pregnancy in renal transplant recipients: An Iranian experience with a report of triplet pregnancy. Transplant Proc 1997;29:2775.
- Russell G, Graveley R, Seid J, Al-Humidan AK, Skjodt H. Mechanisms of action of cyclosporine and effects on connective tissue. SemArthrtis Rheum 1992;21:16-22.
- Moon JI, Park SG, Cheon KO, Kim SI, Kim YS, Park YW. Pregnancy in renal transplant patients. Transplant Proc 2000;32:1869-70.
- Food and Drug Administration. 50573s18.PDF. Page 10. Available from: http://www.fda.gov/cder/ogd/rld/50573s18.pdf [Last accessed on 2006].
 ) and control (oil,
) and control (oil,  ) mouse groups on days
13, 16 and 18 of gestation. *P <0.05
) mouse groups on days
13, 16 and 18 of gestation. *P <0.05