- *Corresponding Author:
- Xingjing Wu
Department of Orthopedics and Traumatology, The First Affiliated Hospital of Wannan Medical College, Yijishan Hospital, Wuhu, Anhui Province 241001, China
E-mail: wuxingjing.123@163.com
| Date of Received | 25 June 2023 |
| Date of Revision | 17 November 2023 |
| Date of Acceptance | 03 June 2024 |
| Indian J Pharm Sci 2024;86(3):1125-1131 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the effect of curcumin treatment on serum inflammatory factors in rats with traumatic arthritis and explores the protective effect of articular surface cartilage in rats with traumatic arthritis. A control group (group A), a model group (group B), and a curcumin group (group C) were randomly chosen. Except for the group A, the curcumin model was established by removing the medial meniscus of the right knee in the other two groups. After the model was successfully established, the group C was intraperitoneally injected with curcumin solution 50 mg/kg, while the group A and B were injected with the same volume of dimethyl sulfoxide solution for 28 d. The levels of serum vascular endothelial growth factor and tumor necrosis factor-alpha and interleukin-1 beta were measured by enzyme-linked immunosorbent assay method. The arthritis index score of the two groups was calculated, the pathological morphology of articular cartilage was observed by hematoxylin and eosin staining, and the apoptosis rate of knee chondrocytes was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling staining. Real-time polymerase chain reaction and Western blot methods were used to detect the expression of autophagy and apoptosis regulatory molecules Beclin-1, B-cell lymphoma 2, caspase-3, messenger ribonucleic acid and protein in knee cartilage. Curcumin can protect the articular surface cartilage of traumatic arthritis rats by reducing inflammatory factors in serum and regulating the apoptosis and autophagy of articular chondrocytes. Curcumin may be a potential drug for the treatment of traumatic arthritis.
Keywords
Curcumin, traumatic arthritis, inflammatory factors, cartilage, synoviocytes
Traumatic Arthritis (TA) refers to the inflammatory reaction and injury of articular cartilage and periarticular tissue caused by joint trauma, which is usually caused by joint trauma, overuse or chronic arthritis[1,2]. In the early stage of TA, abnormal mechanical pressure and disorder of Extracellular Matrix (ECM) stimulate synoviocytes and Matrix Metalloproteinases (MMPs). The destruction of the intact joint surface increases the exposure of CII fibers to MMPs, resulting in erosion of the deep layer of cartilage, resulting in the loss of chondrocytes, and finally subchondral bone exposure[3]. The treatment of TA mainly includes non-drug treatment and drug treatment. The most common first-line treatment is nonsteroidal anti-inflammatory drugs; however they do not reduce pain, do not slow down cartilage degradation, and can cause gastrointestinal complications. For symptomatic knee arthritis, the Osteoarthritis Research Society International (OARSI) and the American College of Rheumatology (ACR) recommend intra-articular injection of corticosteroids. Steroids provide temporary pain relief for patients, but not for underlying causes, and long-term treatment is not recommended. The research of therapeutic drugs has become the focus of attention. With the wide application of traditional Chinese medicine in orthopedic treatment, its value in osteoarthritis is becoming more and more important. Curcumin is a kind of natural polyphenols extracted from the rhizome of turmeric. In recent years, it has been found that curcumin has the effects of anti-tumor, anti-virus, anti-inflammation, anti-oxidation, anti-atherosclerosis, reducing blood lipids and inhibiting angiogenesis. The mechanism of action involves multiple targets[4,5]. Studies have shown that curcumin has a certain therapeutic effect on other inflammation-related diseases, such as rheumatoid arthritis and osteoarthritis[6]. However, there are relatively few studies on curcumin in the treatment of TA. Therefore, this study explored the effect of curcumin on serum inflammatory factors and the protective mechanism of articular surface cartilage in rats with TA.
Materials and Methods
Experimental materials:
Curcumin and Vascular Endothelial Growth Factor (VEGF), Enzyme-Linked Immunosorbent Assay (ELISA) kits (purchased from Shanghai Guyan Experimental Co., Ltd.,) and r-Tumor Necrosis Factor-Alpha (rTNF-α), ELISA kits (purchased from Shenzhen Jingmei Biological Co., Ltd., Batch No: 20060406).
Experimental animal:
Thirty Sprague-Dawley (SD) rats were purchased from Qinglongshan Animal breeding Farm in Jiangning, Nanjing. Certificate: SCXK (Su) 2009-0008. Half male and female, weight (251±50) g, fed with standard feed, free drinking water, room temperature 18°-26°, humidity about 70 %, adaptive feeding for 1 w.
Grouping and modeling:
A control group (group A), a model group (group B), and a curcumin group (group C) were randomly chosen. Rat model of TA was established in group B and C. Under 1 % pentobarbital sodium (110 mg/kg) anesthesia, 2 cm incision was made longitudinally beside the bone of the right knee under sterile conditions, and the knee joint was exposed, the associated ligament was cut and the medial meniscus was resected, and the incision was closed layer by layer after cleaning the wound. After operation, penicillin 200 000 U was injected intramuscularly for 3 d, and the rats were allowed to move freely after operation. 7 d-21 d after modeling, the rats showed stiffness and swelling of the affected limb, accompanied by joint deformity and obvious limitation of movement of the affected limb, i.e. the modeling was successful.
Drug administration method:
The group B and C were injected intraperitoneally on the 7th d of modeling. Curcumin solution was injected intraperitoneally into group C, while Dimethyl Sulfoxide (DMSO) solution was injected intraperitoneally into groups A and B.
Detection of serum VEGF in rats:
After 4 w of treatment, the rats were decapitated and the whole blood of 3 ml was collected and separated into serum. The serum was stored at -20°. Perform related operations according to the ELISA kit instructions provided. The Optical Density (OD) value was determined by enzyme labeling instrument at 450 nm, and the concentration was calculated accordingly.
Inflammatory factors TNF-α and Interleukin-1 Beta (IL-1β):
Follow the instructions provided by the ELISA kit, set a negative control, and react for 9 min at 37° without washing. Biotin-labeled antibodies (TNF-α and IL-1β) were added and incubated for 30 min, then washed for 5 times with 0.01 M Tris-Buffered Saline (TBS). 3,3',5,5'-Tetramethylbenzidine (TMB) chromogenic solution reaction was carried out at 37° for 18-22 min, and TMB terminating solution was added. The OD value was determined by enzyme labeling instrument at 450 nm. Draw the standard curve and calculate the concentration.
Real-time detection:
The mRNA expression levels of B-cell lymphoma 2 (Bcl-2) acting protein 1 (Beclin-1), specific cysteinase caspase-3 and Bcl-2 in articular cartilage were detected. The cells were collected 38 h after transfection, and the total Ribonucleic Acid (RNA) was extracted by Trizol reagent. RNA reverse transcriptional kit was used to synthesize complementary Deoxyribonucleic Acid (cDNA). Polymerase Chain Reaction (PCR) amplification instrument was used for amplification. The PCR reaction conditions were as follows; 95° 30 s, 95° 5 s, 60° 30 s, 72° 30 s, 72° 30 s, the messenger RNA (mRNA) level of the gene to be tested was detected by SYBR Green, the mRNA level was standardized by Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH), and the gene expression was quantitatively analyzed by 2–∆∆Ct method.
Western blot detection:
The articular surface cartilage tissue was taken to detect the expression level of Beclin-1, Bcl-2 and caspase-3 protein. The articular surface cartilage tissue was added to the cell lytic solution to cleave 20 min in ice bath, and the supernatant was taken to quantify each group of proteins by BCA method, Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) electrolysis, electro transfer to Polyvinylidene Difluoride (PDVF) membrane, skim milk blocked for 1 h, first antibody overnight, second antibody blocked for 1 h, and the second antibody was blocked for 1 h. GAPDH was used as the internal reference. The relative contents of Beclin-1, Bcl-2 and caspase-3 proteins in each group were detected.
Hematoxylin and Eosin staining:
Peel off the intact synovial tissue. The cartilage tissue fixed in 10 % formaldehyde solution was made into 5 μm thick sections and stained with H and E. The pathomorphological changes such as the smoothness of cartilage, the number of surface cells and the arrangement of transitional cells were observed by optical microscope (100x).
Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) staining:
The TUNEL staining sections of rat medial condyle of femur were selected and observed under light microscope. The positive cells were usually brown. Using Image-ProPlus6.0 image analysis system, to count the cells, a visual field with uniform distribution was chosen. A total of 1000 chondrocytes were counted.
Statistical method:
Statistical Package for the Social Sciences (SPSS) 2.0 statistical software was used for statistical processing, and the measurement data were expressed by mean±standard deviation (variance x±s). Single factor analysis of variance was used for multi-group mean comparison, Least Significant Difference (LSD) method was used for pairwise comparison, and Tamhane method was used for uneven variance. The difference was statistically significant (p<0.05).
Results and Discussion
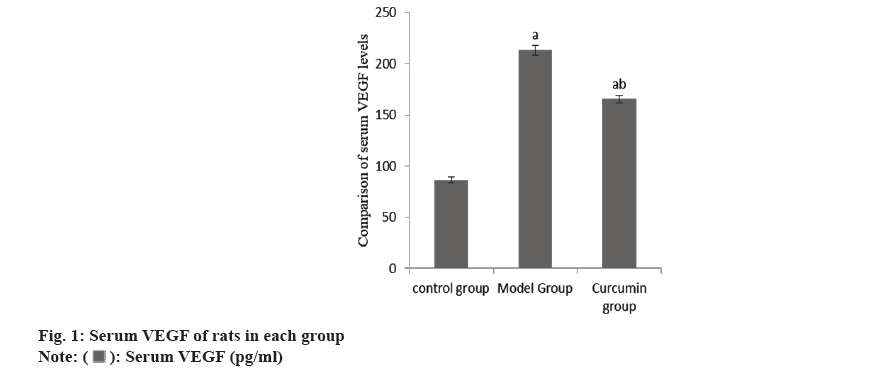
The content of serum VEGF in the group B increased than group A, while this in the group C decreased than group B (Table 1 and fig. 1).
| Group | n | Serum VEGF (pg/ml) |
|---|---|---|
| A | 10 | 86.47±2.58 |
| B | 10 | 213.27±4.87a |
| C | 10 | 165.42±3.71ab |
| F | 2786.86 | |
| p | <0.001 |
Note: ap<0.001 and bp<0.005
Table 1: Serum VEGF of rats.
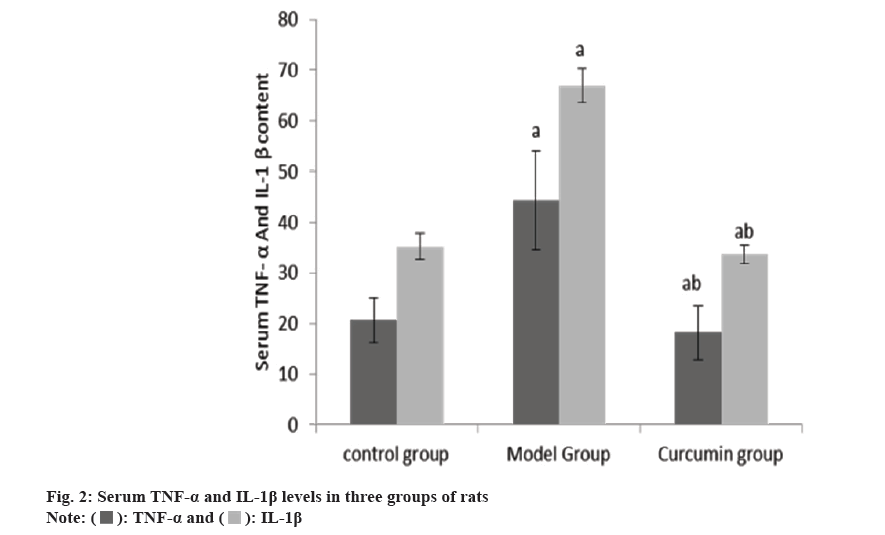
The levels of serum TNF-α and IL-1β in the group B were raised than group A, and these in the group C were reduced than group B (Table 2 and fig. 2).
| Group | n | TNF-α (ng/ml) | IL-1β (ng/ml) |
|---|---|---|---|
| A | 10 | 20.62±4.41 | 35.24±2.52 |
| B | 10 | 44.36±9.67a | 66.98±3.27a |
| C | 10 | 18.24±5.35ab | 33.74±1.85ab |
| F | 44.2 | 516.61 | |
| p | <0.001 | <0.001 |
Note: ap<0.001 and bp<0.005
Table 2: Serum TNF-α and IL-1β levels (x̄±s, ng/l).
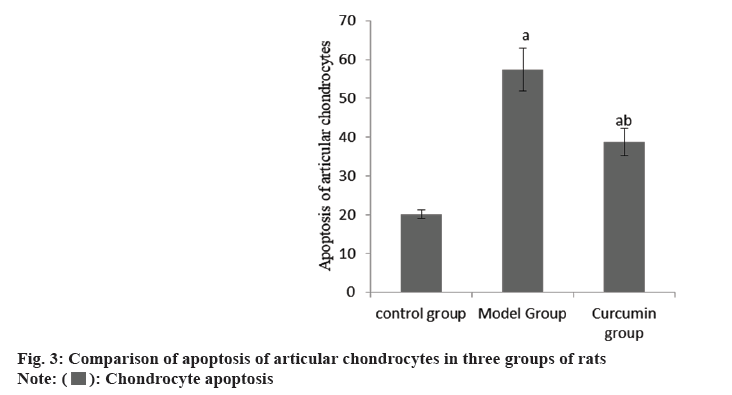
The cartilage surface of the right knee joint of the group A was smooth, that of the group B was seriously damaged, and that of the group C was smooth. The apoptosis rate of articular chondrocytes in the group B was raised than group A, and that in the group C was reduced than group B (Table 3 and fig. 3).
| Group | n | Chondrocyte apoptosis |
|---|---|---|
| A | 10 | 20.14±1.07 |
| B | 10 | 57.38±5.47a |
| C | 10 | 38.75±3.42ab |
| F | 243.23 | |
| p | <0.001 |
Note: ap<0.001 and bp<0.005
Table 3: Apoptosis of articular chondrocytes (x̄±s).
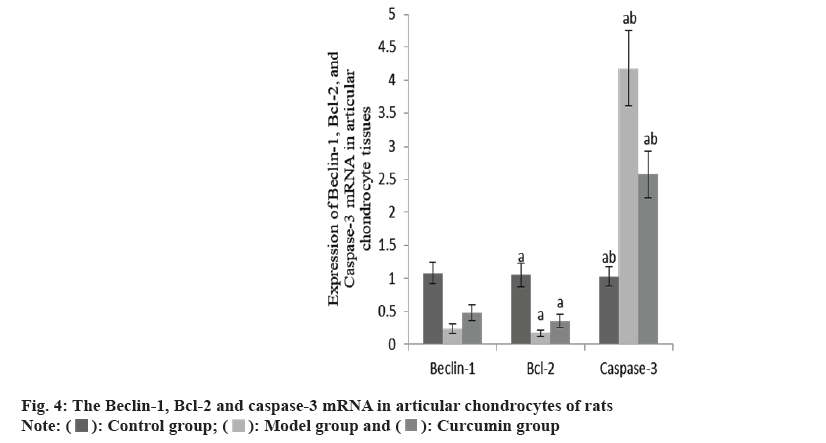
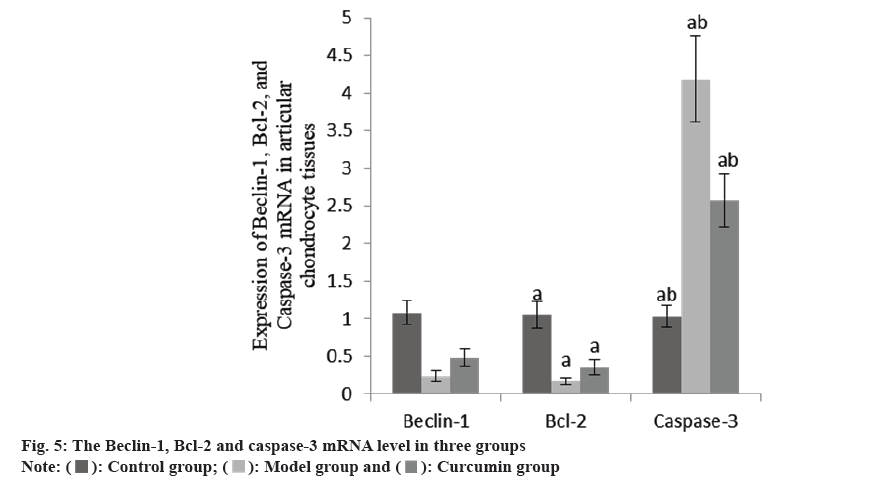
The Beclin-1 and Bcl-2 mRNA in the articular cartilage of the group B were decreased than group A, while these and caspase-3 mRNA increased in the group C than group B (Table 4 and fig. 4).
| Group | n | Beclin-1 | Bcl-2 | Caspase-3 |
|---|---|---|---|---|
| A | 10 | 1.08±0.16 | 1.05±0.18 | 1.03±0.15 |
| B | 10 | 0.24±0.07a | 0.17±0.05a | 4.18±0.57a |
| C | 10 | 0.48±0.12ab | 0.36±0.10ab | 2.57±0.35ab |
| F | 125.08 | 143.27 | 158.40 | |
| p | <0.001 | <0.001 | <0.001 |
Note: ap<0.001 and bp<0.005
Table 4: The Beclin-1, Bcl-2 AND Caspase-3 mRNA in three groups (x̄±s).
Compared with the group A, the Beclin-1 and Bcl-2 protein decreased, while the caspase-3 protein increased in the group B (Table 5 and fig. 5).
| Group | n | Beclin-1 | Bcl-2 | Caspase-3 |
|---|---|---|---|---|
| A | 10 | 1.13±0.10 | 1.05±0.15 | 1.08±0.19 |
| B | 10 | 0.22±0.08a | 0.19±0.09a | 3.84±0.68a |
| C | 10 | 0.44±0.11ab | 0.42±0.12ab | 2.19±0.27ab |
| F | 118.65 | 126.46 | 142.87 | |
| p | <0.001 | <0.001 | <0.001 |
Note: ap<0.001 and bp<0.005
Table 5: The Beclin-1, Bcl-2 and Caspase-3 expression in three groups (x̄±s).
Materia Medica records that turmeric disperses wind, activates blood circulation. It is used in the treatment of liver disease, disperses wind, activates blood circulation and relieves pain. Curcumin is the main active component of turmeric in clinical treatment[7]. The main manifestation of osteoarthritis is articular cartilage degeneration. VEGF is one of the most potent angiogenic factors known to play a key role in the formation of arthritis pannus. Foreign studies have found that the concentration of serum VEGF increases with the progression of arthritis, which is positively correlated with joint index, Lansburg activity index, Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP)[8,9]. The content of serum VEGF in the group B increased than group A and inhibited compared with previous studies, while the content of serum VEGF in the group C decreased compared with the group B. It is suggested that curcumin can significantly reduce VEGF and then inhibit the formation of pannus in arthritis, thus has a protective effect on TA.
IL-1β and TNF-α play critical important role in synovitis and cartilage degradation, indicating that they play a pathological role in inflammatory arthritis. It is reported that by activating NF-κB signal cascade, TNF-α promotes the production of inflammatory mediators, MMPs and chemokines in the inflammatory synovium of RA, thus accelerating bone loss caused by osteoclast formation[10]. In this study, the serum TNF-α and IL-1β in the group B were higher than group A, while the TNF-α and IL-1β in the group C were lower than the group B, indicating that curcumin can reduce the inflammatory response of TA by reducing the release of serum inflammatory factors. At the same time, it was found that the pathological morphology of articular cartilage in the TA group B showed that the cartilage surface was seriously damaged, while in the group C, the cartilage surface was smoother and the apoptosis rate of chondrocytes was lower. It is suggested that curcumin can protect articular cartilage, inhibit apoptosis of chondrocytes and promote the survival of chondrocytes.
Chondrocyte apoptosis is a key factor in cartilage degeneration. The loss of chondrocytes leads to insufficient number of chondrocytes and loss of function. Subsequently, the synthesis of ECM decreases, which may eventually lead to the emergence of osteoarthritis. Therefore, the inhibition of chondrocyte apoptosis may be helpful to the treatment of osteoarthritis. Autophagy is a cellular stress response to exogenous or endogenous stimuli, which can inhibit apoptosis and improve cell survival rate[11]. Beclin-1 is a homologue of yeast ATG6. By forming a complex with Phosphoinositide 3-Kinases (PI3Ks), it can promote the formation of autophagy and induce autophagy[12]. Recent studies have shown that under low nutritional conditions, the apoptosis of chondrocytes is inhibited due to the stimulation of autophagy[13]. Carames et al.[14] found that rapamycin can significantly up-regulate the expression of Beclin-1, LC3-I/II and ATG, thus inhibit the apoptosis of chondrocytes and play a protective role. At the same time, some studies have found that overexpression of Beclin-1 can increase the vitality of chondrocytes and reduce apoptosis. In this study, it was found that the levels of Beclin-1 and Bcl-2 mRNA and protein in cartilage tissue of group C were higher, while the caspase-3 mRNA and protein were lower. It is suggested that curcumin may protect chondrocytes by regulating the expression of autophagy and apoptosis regulatory factors.
To sum up, curcumin can protect the articular surface cartilage of TA rats by reducing the release of serum inflammatory factors and regulating the apoptosis and autophagy of articular chondrocytes. Therefore, curcumin may be a potential drug for the treatment of TA.
Conflict of interests:
The authors declared no conflict of interests.
References
- Peng Y, Yang Z, Li J, Liu S. Research progress on nanotechnology of traditional Chinese medicine to enhance the therapeutic effect of osteoarthritis. Drug Deliv Transl Res 2024;14(6):1517-34.
[Crossref] [Google Scholar] [PubMed]
- McGregor PC, Meldau JE, Liskutin T, Kelly RF, Levack AE, Cohen J, et al. Hospital transfer and delayed reduction of traumatic hip dislocations. Arch Orthop Trauma Surg 2023;143(8):4785-91.
[Crossref] [Google Scholar] [PubMed]
- Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radical Biol Med 2019;132:73-82.
[Crossref] [Google Scholar] [PubMed]
- Pape E, Parent M, Pinzano A, Sapin-Minet A, Henrionnet C, Gillet P, et al. Rapamycin-loaded poly (lactic-co-glycolic) acid nanoparticles: Preparation, characterization, and in vitro toxicity study for potential intra-articular injection. Int J Pharm 2021;609:121198.
[Crossref] [Google Scholar] [PubMed]
- Mohamed Z, El-Kader AE, Salah-Eldin AE, Mohamed O, Awadalla EA. Protective effects of curcumin against acetamiprid-induced neurotoxicity in male albino rats. Biol Bull 2023;50(4):509-21.
[Crossref] [Google Scholar] [PubMed]
- Shen XY, Li Y, Zhang Z. Research progress of curcumin in the treatment of osteoarthritis. Zhonghua Wai Ke Za Zhi 2021;59(6):554-7.
[Google Scholar] [PubMed]
- Wang T, Hou Z, Chen X, Zhao L, Zhu D, Wang N, et al. Analysis of the medication rules of Traditional Chinese Medicines (TCMs) in treating liver cancer and potential TCMs exploration. Pharmacol Res Mod Chin Med 2022;3:100086.
- Ma K, Zhang Y, Hao J, Zhao J, Qi Y, Liu C. Correlation analysis of systemic immune inflammatory index, serum IL-35 and HMGB-1 with the severity and prognosis of sepsis. Pak J Med Sci 2023;39(2):497.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Wang Y, Guo R. NKD2 is correlated with the occurrence, progression and prognosis of thyroid carcinoma. Eur J Med Res 2022;27(1):235.
[Crossref] [Google Scholar] [PubMed]
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014;2014.
[Crossref] [Google Scholar] [PubMed]
- Jakhar R, Paul S, Bhardwaj M, Kang SC. Astemizole-Histamine induces Beclin-1-independent autophagy by targeting p53-dependent crosstalk between autophagy and apoptosis. Cancer Lett 2016;372(1):89-100.
[Crossref] [Google Scholar] [PubMed]
- Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, et al. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev Cell 2016;37(4):337-49.
[Crossref] [Google Scholar] [PubMed]
- Alvarez-Garcia O, Olmer M, Akagi R, Akasaki Y, Fisch KM, Shen T, et al. Suppression of REDD1 in osteoarthritis cartilage, a novel mechanism for dysregulated mTOR signaling and defective autophagy. Osteoarthr Cartil 2016;24(9):1639-47.
[Crossref] [Google Scholar] [PubMed]
- Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum 2010;62(3):791-801.
[Crossref] [Google Scholar] [PubMed]
 .
.
 .
.
 .
.
 .
.
 .
.