- *Corresponding Author:
- Min Li
Department of Vascular Surgery, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710061, China
E-mail: limin2021_2023@163.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “215-221” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effects of cognitive behavioral therapy on the clinical outcomes and self-management behaviors of patients with diabetic peripheral neuropathy treated with prostilbestrol. 204 patients with diabetic peripheral neuropathy treated with prostilbestrol from January 2022 to January 2023 were included in the study and randomly divided into a control group (n=102, receiving usual care) and a cognitive behavioral therapy group (n=102, adding cognitive behavioral therapy to usual care) to compare the intervention effects of the two groups. All summary diabetes self-care activities scores as well as the motor nerve conduction velocity and sensory nerve conduction velocity of the median and common peroneal nerves were higher after the intervention in both groups, with the cognitive behavioral therapy group outperforming the control group (p<0.05). Following the intervention, both groups' ratings on the self-assessment scales for anxiety, sadness, and quality of life in Norfolk diabetic neuropathy dropped, with the cognitive behavioral therapy group scoring lower than the control group (p<0.05). Cognitive behavioral therapy has considerable impacts on diabetic peripheral neuropathy patients who are receiving prostilbestrol treatment, including improvements in their neurophysiological condition, self-management skills, reduction of anxiety and sadness, and quality of life.
Keywords
Diabetic peripheral neuropathy, cognitive behavioral therapy, self-management, anxiety, depression, quality of life
A common long-term effect of diabetes, Diabetic Peripheral Neuropathy (DPN) is characterized by symmetrical pain and sensory anomalies. It can affect sensory, motor and vegetative neurons[1]. According to statistics, the number of people with diabetes is currently 420 million worldwide and is expected to reach 560 million by 2030[2]. China has 114 million adults with diabetes, the highest number of adults with diabetes[3]. Studies have shown that the incidence of DPN in diabetic patients is as high as 51.40 %[4], although the disease’s pathophysiology is still unknown, the majority of researchers think that vascular damage, metabolic problems, cytokine abnormalities and other variables are involved, and the disease is insidious and irreversible in the late stage of development, and can develop into diabetic plantar ulcers, diabetic foot and so on for a long time without improvement, increasing the risk of amputation, seriously affecting the patient's life and increasing the risk of amputation and cause a serious psychological and medical burden to the patient[5,6].
Prostil is currently the main clinical treatment for DPN. It is a prostaglandin-like substance with significant vasodilating effects and can effectively regulate lipid metabolism, inhibit abnormal lipid synthesis in nerve myelin sheaths, block further damage to nerve fiber myelin sheaths, effectively improve blood flow to the nerve endothelium, and play a nerve-nourishing role in the treatment of DPN[7]. However, the clinical benefits of prostilbestrol in the treatment of DPN have been overestimated to some extent, while the susceptibility to side effects has been ignored[8]; and in addition to pharmacological treatment, good psychological status and quality self-management level are also important elements in the treatment of DPN. Patients with DPN have a heavy psychological burden and often experience anxiety, depression and other undesirable emotions, and their acceptance of psychological treatment is higher than that of pharmacological treatment[9], and the psychological aspect of promotion depends on the facilitation of quality and efficient nursing measures. Conventional care starts with the disease itself and pays less attention to the psychological and cognitive aspects of the patient. Cognitive Behavioral Therapy (CBT) emphasizes the crucial influence of a person's cognition on their emotions and behavior, and is a group of brief psychotherapies that aim to change poor cognition and eliminate negative emotions and behaviors by altering thinking or beliefs and behavior[10,11]. CBT has been shown to be an effective therapy to reduce distress[12], reduce emotional problems[13], improve adherence[14] and control blood glucose levels through adherence[15], but it is effective in DPN. However, the observation of its effects in DPN has not been reported. We believe CBT plays a substantial role in the treatment of DPN patients receiving prostilbestrol, greatly strengthening the patients' self-management behaviors.
To test these hypotheses, we prospectively conducted a study on the effect of CBT on the clinical outcomes and self-management behaviors of patients with DPN treated with prostilbestrol, with the aim of informing the clinical treatment and care of DPN.
Materials and Methods
Experimental design:
In the trial, 204 DPN patients receiving prostil from January 2022 to January 2023 were included. They were randomly assigned to one of two groups; CBT (n=102), getting standard treatment or the control group (n=102), adding CBT to usual care. In fig. 1, the study flow is depicted. All study participants signed an informed consent form, which was authorized by our hospital’s medical ethics committee. All study participants signed informed consent forms after our medical ethics committee authorized the study.
Inclusion and exclusion criteria:
Inclusion criteria: Patients met the diagnostic criteria for DPN[16]; had an abnormal neurophysiological examination and had unilateral or symmetrical sensory abnormalities, loss of superficial sensation or diminished sensation in the extremities.
Exclusion criteria: Patients with cardiac, hepatic or renal abnormalities; patients with severe hematological and neurological disorders; patients with acute complications of diabetes mellitus; patients during pregnancy or lactation; patients with peripheral neuropathy due to lumbar spine pathology or vasculitis; patients with cognitive and mental impairment, and patients with allergy to prostilbestrol.
Methods:
Patients in both groups received exercise and diet control, blood pressure and blood glucose control after admission to hospital. Prostil injection (Beijing Tide Pharmaceutical Co., Ltd, approval number-State Drug quantification H10980023, specification of 1 ml:5 μg) 10 μg was added to 100 ml of saline for intravenous infusion, 1 time/day, for 14 d.
The control group received routine care, mainly covering blood pressure, blood glucose and other indicators monitoring; health education; medication guidance and diet and exercise guidance, etc.
The CBT group added CBT to the control group in three phases; initial, intermediate and final, using group therapy, individual therapy combined with special cases, 1 h/time, 1 time/week, for a total of 12 w.
Initial phase: The nurse builds a strong therapeutic bond and relationship with the patient, speaks with them frequently, and makes sure they are willing to cooperate and actively participate. On this basis, the patient's basic situation is fully assessed, including life experiences, life beliefs, assumptions, expectations, rules and attitudes, etc. Emphasis is placed on understanding the patient's psychological state and encouraging the patient to take the initiative to communicate and confide in the health care staff. In order to entirely reduce the patient's worry, depression, and other negative emotions, they carefully listen to the patient's primary complaint during this time and encourage the patient to face the condition with a positive and hopeful attitude and to build up confidence in defeating the sickness. In order to totally alleviate the patient’s negative feelings, such as anxiety and depression, they listen to the patient’s concerns and urge them to fight the sickness with a positive attitude and build up confidence in defeating the disease.
Intermediate stage: To educate patients on the principles of CBT and help they understand the relationship between negative automatic thinking, imagery behaviors and the illness. Patients are encouraged to identify, observe and evaluate their negative automatic thinking in a structured way, re-evaluate their beliefs about the illness and their lives, and help them to develop new ways of perceiving and correcting previous erroneous cognitive biases. Together with the patient, goals and plans for treatment are formulated, poor behaviors patterns are corrected, and patients are helped to develop scientific habits, especially in terms of diet, and to establish scientific ways of coping. Patients are also introduced to cases of DPN treatment with good results and long-term normal survival, and encouraged to learn from their treatment experience. Patients are encouraged to monitor automatic thoughts at the end of treatment to increase motivation to participate in treatment. Summarizing and reinforcing the treatment planning sadness at final stage.
Assessment indicators:
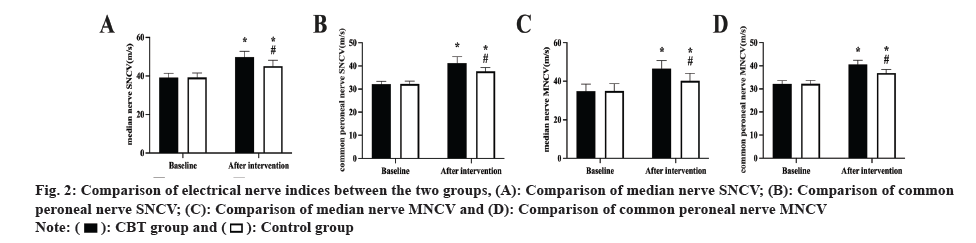
Neurophysiological indicators: After measuring the median and common peroneal nerves Motor Nerve Conduction Velocity (MNCV) and Sensory Nerve Conduction Velocity (SNCV) before and after the intervention in both groups, the measurements were repeated three times, and the final scores were averaged.
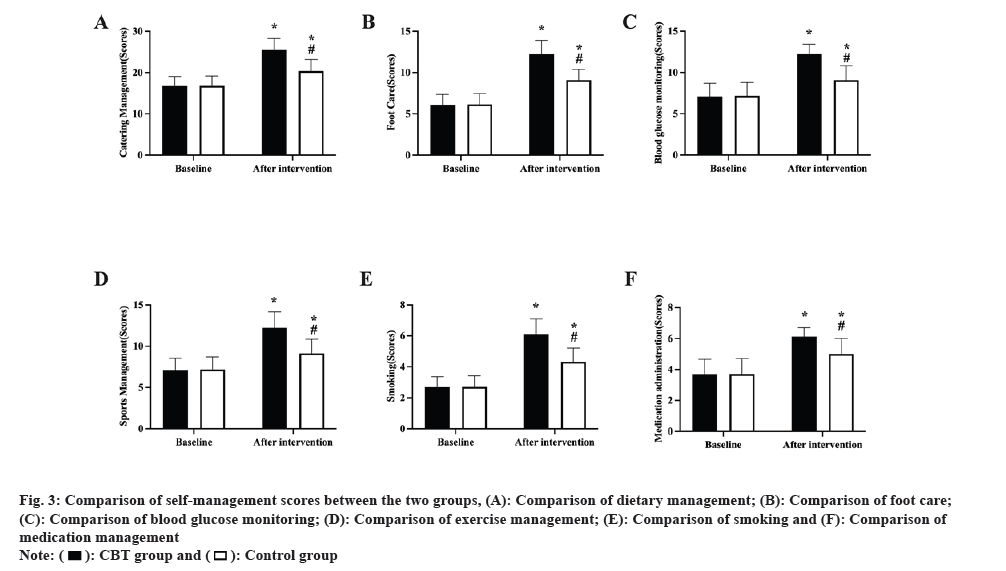
Self-management scores: The Summary Diabetes Self Care Activities (SDSCA) was used to evaluate self-management behaviors in both groups before and after the intervention[17]. The scale consists of 11 entries, each rated 0 to 7 on six dimensions; diet management (0 to 28), foot care (0 to 14), blood glucose monitoring (0 to 14), exercise management (0 to 14), smoking (0 to 7), and medication management (0-7 points), with higher scores indicating higher levels of self-management.
Anxiety and depression scores: Before and after the intervention, the anxiety Self-Assessment Scale (SAS) and the Self-Rating Depression Scale (SDS) were used to measure the unpleasant emotions in both groups. 20 items were included in the SAS score, including anxiety, insanity, panic and fear, with a total score of 80. The SDS score consists of 20 items including depression, palpitations, sleep disturbance, crying, loss of appetite, etc. The overall score is 80, where a score of less than 53 indicates normal functioning, a score of between 53 and 62 indicates mild depression, a score of between 63 and 72 indicates moderate depression, and a score of greater than 73 indicates severe depression.
Quality of life scores: The Norfolk Quality of Life questionnaire Diabetic Neuropathy (QOL-DN) has 35 entries in five areas and was used to measure quality of life in both groups before and after the intervention[18]. Daily activities (0-20 points), physical functioning (4-56 points), small-fiber (0-16 points), autonomic neuropathy (0-12 points), and symptoms (0-32 points) for a total score of 4-136 points. Higher scores indicate a lower quality of life.
Statistical analysis:
GraphPad Prism 9.0 was used for the graphing and Statistical Package for the Social Sciences (SPSS) 21.0 for the analysis. Measurement data were expressed as (x±s) and compared using t-test, Analysis of Variance (ANOVA), and Least Significant Difference (LSD) test; count data were expressed as [n (%)] and compared using the Chi-square (χ²) test. At p<0.05, differences were declared statistically significant.
Results and Discussion
Demographic and basic clinical information was similar in both groups (p>0.05, Table 1). The median and common peroneal nerve’s MNCV and SNCV did not differ between the two groups prior to the intervention (p>0.05); during the intervention, both group’s MNCV and SNCV rose, with the CBT group having a bigger rise than the control group (p<0.05, fig. 2).
| Variable | CBT group (n=102) | Control group (n=102) | χ² or t | p |
|---|---|---|---|---|
| Age (years) | 56.39±4.31 | 56.45±4.27 | 0.010 | 0.921 |
| Male/female (n) | 57/45 | 55/47 | 0.079 | 0.778 |
| Duration of diabetes (months) | 95.49±4.97 | 95.68±4.88 | 0.276 | 0.783 |
| Body mass index (kg/m²) | 29.58±2.28 | 29.67±2.21 | 0.286 | 0.775 |
| Fasting Blood Sugar (mg/dl) | 165.48±10.29 | 166.03±11.02 | 0.368 | 0.713 |
| 2 h postprandial glucose (mg/dl) | 216.59±16.59 | 217.08±17.03 | 0.208 | 0.835 |
| Glycated hemoglobin A1c (%) | 8.83±1.21 | 8.89±1.25 | 0.348 | 0.728 |
Table 1: Demographic and basic clinical information of the two groups.
All SDSCA scores in the two groups were equal before the intervention (p>0.05); after the intervention, all SDSCA scores were higher in both groups, with the CBT group outperforming the control group (p<0.05, fig. 3).
Prior to the session, there was no difference between the two group’s SAS and SDS scores (p>0.05); during the intervention, SAS and SDS scores declined in both groups, but were lower in the CBT group than in the control group (p<0.05, fig. 4).
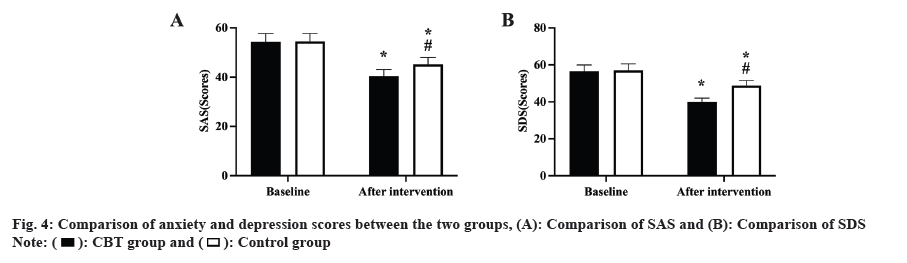
Prior to the intervention, there was no change in any of the QOL-DN scores between the two groups (p>0.05); following the intervention, both group’s QOL-DN scores dropped, but the CBT group's scores were lower than the control group's (p<0.05, fig. 5).
Our study’s findings demonstrated that the CBT group’s self-management scores were significantly higher than those of the control groups, demonstrating that CBT can successfully enhance DPN patient’s self-management behavior. The low level of self-management among DPN patients in general may be related to less awareness of the disease, reliance on medication alone for treatment, and poor health management concepts. CBT has been shown to be effective in improving the self-management level of hemodialysis patients in terms of diet and water intake[19]. In this study, CBT was applied to DPN patients to actively help them identify and improve their behavioral patterns, promote their cognitive reconstruction, increase their correct knowledge of DPN, and make them realize the importance of self-management for disease control. The benefits of self-management behaviors can be seen in improved medication adherence, regular blood glucose monitoring, and the establishment of good exercise habits[20]. The benefits of self-management behaviors are reflected in the optimization of neurophysiological indicators in the laboratory.
Additionally, our results demonstrated that the SAS and SDS scores were lower in the CBT group than in the control patients, demonstrating the effectiveness of CBT in reducing negative feelings in DPN patients. CBT has been shown to be effective in the treatment of depression in various settings[21,22], demonstrating its excellent heart optimization and mood relieving effects. Also, some clinical studies have shown that CBT reduces the emotional burden and increases the resilience of patients with diabetes[23]. CBT can help patients develop positive attitudes and strengthen functional outcomes through individual and group therapy, reflecting the importance of CBT as an effective intervention for DPN. An effective intervention for DPN[24,25].
According to our research, CBT therapies improved the quality of life in DPN patients. Quality of life is one of the current indicators to be improved when patients receive clinical treatment and care, and is a key indicator of the effectiveness of clinical information and care. CBT has been shown to improve quality of life in adults with type 2 diabetes[26]. In patients with DPN, CBT has been shown to improve overall quality of life by enhancing self-management behaviors, improving negative emotions and optimizing neurophysiological indicators in both physical and psychological dimensions.
There are a number of limitations to this study due to objective constraints. First, the small number of cases included leads to a certain degree of statistical calculation chance; and fewer older patients were included, who may have more severe DPN problems. Secondly, the study focused on the neurophysiological indicators, and anxiety and depression of patients with DPN with behavioral cognitive therapy, and many other factors, such as patients' coping mechanisms and emotional support systems, were not looked at in this research. Future research needs to address these limitations.
CBT can effectively improve the neurophysiological status of DPN patients treated with prostilbestrol, strengthen their self-management ability, reduce anxiety and depression, and improve the quality of life, which is significant and has high value for clinical application and promotion.
Conflict of interests:
The authors declared no conflict of interests.
References
- Diabetes Society of Chinese Medical Association. Guidelines for the prevention and treatment of type 2 diabetes in China (2017). Chin J Pract Intl Med 2018;4:292-344.
- Zhu D. A decade of sharpening the sword: Progress and reflections on diabetes research in China (III). Chin J Diabetes 2019;11(3):145-8.
- Federation ID. International diabetes federation: IDF diabetes atlas. 8th ed. Brussels, Belgium; 2013.
- Wang L, Bai H, Han M. Prevalence of chronic complications in elderly patients with type 2 diabetes mellitus and analysis of related factors. Mod Chin J 2018;56(26):22-6.
- Happich M, John J, Stamenitis S, Clouth J, Polnau D. The quality of life and economic burden of neuropathy in diabetic patients in Germany in 2002—results from the Diabetic Microvascular Complications (DIMICO) study. Diabetes Res Clin Pract 2008;81(2):223-30.
[Crossref] [Google Scholar] [PubMed]
- Venkataraman K, Wee HL, Leow MK, Tai ES, Lee J, Lim SC, et al. Associations between complications and health-related quality of life in individuals with diabetes. Clin Endocrinol 2013;78(6):865-73.
[Crossref] [Google Scholar] [PubMed]
- Liu LX, Liang XM, Wu MD. Clinical study of beraprost combined with hyperbaric oxygen for the treatment of diabetic peripheral neuropathy. Chin J Clin Pharmacol 2016;32(18):1657-9.
- Fava GA. Rational use of antidepressant drugs. Psychother Psychosom 2014;83(4):197-204.
[Crossref] [Google Scholar] [PubMed]
- McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs. pharmacologic treatment of psychiatric disorders: A meta-analytic review. J Clin Psychiatry 2013;74(6):13979.
[Crossref] [Google Scholar] [PubMed]
- Creswell C, Hentges F, Parkinson M, Sheffield P, Willetts L, Cooper P. Feasibility of guided cognitive behaviour therapy (CBT) self-help for childhood anxiety disorders in primary care. Mental Health Family Med 2010;7(1):49-57.
[Google Scholar] [PubMed]
- Tarrier N. Cognitive behavior therapy for schizophrenia and psychosis: Current status and future directions. Clin Schizophr Relat Psychoses 2010;4(3):176-84.
- Andreae SJ, Andreae LJ, Richman JS, Cherrington AL, Safford MM. Peer-delivered cognitive behavioral training to improve functioning in patients with diabetes: A cluster-randomized trial. Ann Family Med 2020;18(1):15-23.
[Crossref] [Google Scholar] [PubMed]
- Ciharova M, Furukawa TA, Efthimiou O, Karyotaki E, Miguel C, Noma H, et al. Cognitive restructuring, behavioral activation and cognitive-behavioral therapy in the treatment of adult depression: A network meta-analysis. J Consult Clin Psychol 2021;89(6):563.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Li Z, Sun J. Effects of cognitive behavioral therapy–based intervention on improving glycaemic, psychological and physiological outcomes in adult patients with diabetes mellitus: A meta-analysis of randomized controlled trials. Front Psychiatry 2020;11:711.
[Crossref] [Google Scholar] [PubMed]
- Pan X, Wang H, Hong X, Zheng C, Wan Y, Buys N, et al. A group-based community reinforcement approach of cognitive behavioral therapy program to improve self-care behavior of patients with type 2 diabetes. Front Psychiatry 2020;11:719.
[Crossref] [Google Scholar] [PubMed]
- Electromyography and clinical neurophysiology group of the Chinese medical association neurology branch, neuromuscular disease group of the Chinese medical association neurology branch. Consensus on the diagnosis and treatment of diabetic peripheral neuropathy. Chin J Neurol 2013;46(11):787-9.
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care 2000;23(7):943-50.
[Crossref] [Google Scholar] [PubMed]
- Vinik EJ, Vinik AI. Transcending tradition: Quality of life as the inextricable link between activities of daily living and specific organ and disease states. Res Hum Capital Dev 2008;9(16):29-52.
- Yang YN, Qian HY, Tang LX. Effects of group cognitive behavioral therapy combined with dietary intervention on psychological and residual renal function in hemodialysis patient. J Nurs 2014;29(23):58-61.
- Dobson D, Dobson KS. Evidence-based practice of cognitive-behavioral therapy. New York, Guilford Press; 2009.
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clin Psychol Rev 2006;26(1):17-31.
[Crossref] [Google Scholar] [PubMed]
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognit Ther Res 2012;36:427-40.
[Crossref] [Google Scholar] [PubMed]
- Harvey AG, Dong L, Bélanger L, Morin CM. Mediators and treatment matching in behavior therapy, cognitive therapy and cognitive behavior therapy for chronic insomnia. J Consult Clin Psychol 2017;85(10):975.
[Crossref] [Google Scholar] [PubMed]
- van der Feltz-Cornelis C, Allen SF, Holt RI, Roberts R, Nouwen A, Sartorius N. Treatment for comorbid depressive disorder or subthreshold depression in diabetes mellitus: Systematic review and meta-analysis. Brain Behav 2021;11(2):e01981.
[Crossref] [Google Scholar] [PubMed]
- Lu X, Yang D, Liang J, Xie G, Li X, Xu C, et al. Effectiveness of intervention program on the change of glycaemic control in diabetes with depression patients: A meta-analysis of randomized controlled studies. Primary Care Diabetes 2021;15(3):428-34.
[Crossref] [Google Scholar] [PubMed]
- Noroozi Z, Hamidian S, Khajeddin N, Honarmand MM, Zargar Y, Rashidi H, et al. Improving depression and quality of life in patients with type 2 diabetes: Using group cognitive behavior therapy. Iran J Psychiatry 2017;12(4):281.
[Google Scholar] [PubMed]
 .
.
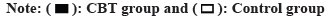 .
.
 .
.
 .
.



