- *Corresponding Author:
- Qin Tang
Department of Gynaecology and Obstetrics, Zhejiang Xinda Hospital, Huzhou, Zhejiang 313000, China
E-mail: 75052798@qq.com
| Date of Received | 04 December 2021 |
| Date of Revision | 10 July 2022 |
| Date of Acceptance | 19 January 2023 |
| Indian J Pharm Sci 2023;85(1):76-82 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect and mechanism of dexmedetomidine on SKOV3 proliferation, migration and invasion in ovarian cancer cell strains and the effect on high mobility group protein A2 expression. Western blot was used to detect the protein expression of high mobility group protein A2 in SKOV3 ovarian cancer cells; control group (without any treatment), (dexmedetomidine group 100 ng/ml was treated for 24 h), si-control group (transfection with si-control), si-high mobility group protein A2 group (transfection with si-high mobility group protein A2), dexmedetomidine+plasmid cloning deoxyribonucleic acid group (after transfection with plasmid cloning deoxyribonucleic acid group followed by dexmedetomidine 100 ng/ml treated for 24 h), dexmedetomidine+plasmid cloning deoxyribonucleic acid group-high mobility group protein A2 group (after transfection with plasmid cloning deoxyribonucleic acid-high mobility group protein A2 followed by dexmedetomidine 100 ng/ml treated for 24 h), were all transfected into SKOV3 cells using Lipofectamine; the proliferation of cells was detected by methyl thiazolyl tetrazolium assay; cell migration and invasion were detected by transwell assay; high mobility group protein A2 expression was measured by quantitative reverse transcription-polymerase chain reaction. The proliferation, migration and invasion of SKOV3 cells treated with dexmedetomidine were significantly down-regulated and high mobility group protein A2 expression was significantly down-regulated compared with the control group (p<0.05); knockdown of high mobility group protein A2 significantly inhibited SKOV3 cell proliferation, migration and invasion and overexpression of high mobility group protein A2 reversed the inhibitory effects of dexmedetomidine on ovarian cancer cell proliferation, migration and invasion. Dexmedetomidine can inhibit the proliferation, migration and invasion of ovarian cancer cells SKOV3 and the mechanism may be related to the direct down-regulation of high mobility group protein A2, which will provide a theoretical basis for dexmedetomidine treatment of ovarian cancer.
Keywords
Dexmedetomidine, high mobility group protein A2, ovarian cancer, proliferation, migration
Ovarian cancer is a common gynecological malignancy, the incidence of which is second only to cervical cancer and the mortality rate ranks first among female neoplasms, seriously threatening women’s physical and mental health[1]. Ovarian cancer is highly susceptible to drug resistance during the later course of treatment and although new drugs are continuously influx to the market, there are still patients who are insensitive to the therapeutic agents[2]. Dexmedetomidine (DEX) is novel highly selective alpha (α) 2 adrenergic receptor agonists with sedative, analgesic, anxiolytic, and oxygen consumption lowering effects in the myocardium[3]. Recently, DEX was found to effectively inhibit the stress response in the perioperative period and relieve the suppression of postoperative cellular immune function in breast cancer patients[4], but the role and mechanism in ovarian cancer are still unclear. Members of the High Mobility Group Protein A (HMGA) family are expressed abundantly in the early embryo and include HMGA1, HMGA2[5]. The HMGA1 gene is mainly expressed in proliferating epithelial cells and areas of parenchymal organ tissue, whereas the HMGA2 gene is highly expressed in all mesenchymal cell condensations and mesenchymal derivatives[6]. HMGA2 may be completely silenced in adult tissues, but its expression is observed in many human malignancies, including ovarian cancer[7]. The aim of this study was to investigate the mechanisms underlying the effects of DEX on the proliferation, migration and invasion of ovarian cancer cells.
Materials and Methods
Materials:
The human ovarian cancer cell strain SKOV3 was purchased from American Type Culture Collection (ATCC); DEX (batch No.: 113775-47-6) was purchased from the Yangtze River Pharmaceutical Group; Dulbecco's Modified Eagle Medium (DMEM) and Methyl Thiazolyl Tetrazolium (MTT) were purchased from GIBCO, USA; Lipofectamine™ 2000 was purchased from Shanghai Sunshine Biotechnology Co., Ltd; Polyvinylidene Fluoride (PVDF) membranes were purchased from Roche Diagnostics GmbH, Germany; Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) kit and Electrochemiluminescence (ECL) luminescent solution were purchased from Beyotime Biotechnology; HMGA2 monoclonal antibody was purchased from Shanghai Xinyu Biotechnology Co., Ltd; horseradish peroxidase labeled secondary antibodies were purchased from Beijing Bersee Science and Technology Co., Ltd. All plasmids and primers were designed and synthesized by Shanghai Gene Pharm Co., Ltd.
Methods:
Cell culture: SKOV3 cells were cultured with DMEM (with 10 % fetal bovine serum) and routinely grown in an incubator at 37°, 5 % Carbon dioxide (CO2).
Cell transfection: SKOV3 cells were treated with DEX at concentrations of 0 and 100 ng/ml for 24 h, which were labeled as Control (Con) group and DEX group respectively. si-con, si-HMGA2, plasmid cloning Deoxyribonucleic Acid (pcDNA) group and pcDNA-HMGA2 were transfected into SKOV3 cells following the Lipofectamine™ 2000 protocol, labeled as si-con group, si-HMGA2 group, pcDNA group and pcDNA-HMGA2 group; after transfection of pcDNA and pcDNA-HMGA2 into SKOV3 cells and treatment with 100 ng/ml DEX for 24 h, the cells were labeled as DEX+pcDNA group, DEX+pcDNA-HMGA2 group and the efficiency of low expression or overexpression was verified by quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) and SKOV3 cells were subsequently collected for subsequent assays.
qRT-PCR experiments: Grouping SKOV3 cells were collected, Ribonucleic Acid (RNA) was extracted following the instructions of RNA extraction kit and complementary DNA (cDNA) preparation and amplification reactions were performed by reverse transcription kit, qRT-PCR kit protocol from Takara Co., Ltd. Dalian, China, respectively. HMGA2 expression was finally calculated as 2-ΔΔCt. HMGA2 upstream primer 5′-CTCAAAAGAAAGCAGAAGCCACTG-3′, reverse primer 5′-TGAGCAGGCTTCTTCTGAACAACT-3′; the internal reference glyceraldehyde 3-phosphate dehydrogenase was used as the universal primer. All primers were synthesized by Shanghai GenePharma Co., Ltd.
Western blot experiments: Grouping SKOV3 cells in cell transfection were collected, total protein was extracted in the lysate, proteins were quantified by Bicinchoninic Acid (BCA) and bath denatured in boiling water. Next, protein electrophoresis and transmembrane were performed; after blocking with 5 % nonfat dry milk for 2 h at room temperature, the membranes were washed with the addition of primary antibody (HMGA2 monoclonal antibody, 1:1000), overnight incubation at 4°, added secondary antibody (horseradish peroxidase labeled immunoglobulin G Heavy and Light (H&L) chains, 1:2000) and incubation at 37° for 2 h, washed the membranes. Add luminescent solution and expose.
MTT experiments: SKOV3 cells were treated with 0 ng/ml, 50 ng/ml, 100 ng/ml, 200 ng/ml and 20 μl, 5 g/l MTT solution was added at 24 h, 48 h, 72 h and was reacted for 4 h and 150 μl Dimethyl Sulfoxide (DMSO) was added and the SKOV3 cell absorbance (Optical Density (OD)) after crystal dissolution was detected at a wavelength of 490 nm. Grouping SKOV3 cells in cell transfection also detected SKOV3 cell absorbance (OD).
Transwell chamber assay for cell migration and invasion: In the upper chamber of a transwell chamber coated with (to detect invasion) or without (to detect migration) Matrigel, 100 μl cell transfection grouped SKOV3 cells (105/ml serum-free medium) were added and 600 μl of serum containing medium was added in the lower chamber and incubated for 24 h. SKOV3 cells on the lower surface of the chamber were fixed with methanol for 30 min, stained with 0.1 % crystal violet and after 20 min, three fields were randomly selected, photographed under a microscope and the invaded or migrated cells were counted and the average value was taken as the number of invaded or migrated cells.
Statistical treatment:
All data in the experiments were analyzed using Statistical Package for the Social Sciences (SPSS) 21.0 software. The measurement data were expressed as the mean±standard deviation (x̄±s) and data comparisons among multiple groups, pairwise comparisons were performed using one-way Analysis of Variance (ANOVA) and t-test and a value of p<0.05 was considered statistically significant.
Results and Discussion
Results are as shown in Table 1, SKOV3 ovarian cancer cells in the 50 ng/ml, 100 ng/ml and 200 ng/ml groups exhibited significantly decreased cell proliferation compared with the 0 ng/ml group at 48 h and 72 h (p<0.05). A concentration of 100 ng/ml was chosen for subsequent experiments.
| Group | OD 490 nm | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 0 ng/ml | 0.46±0.05 | 0.68±0.07 | 1.16±0.10 |
| 50 ng/ml | 0.41±0.03 | 0.54±0.06* | 0.96±0.09* |
| 100 ng/ml | 0.39±0.04 | 0.48±0.06* | 0.82±0.09* |
| 200 ng/ml | 0.37±0.04 | 0.45±0.05* | 0.75±0.08* |
| F | 2.712 | 8.568 | 12.034 |
| p | 0.115 | 0.007 | 0.003 |
Note: *p<0.05 compared with 0 ng/ml group
Table 1: Proliferation Effects of DEX on Ovarian Cancer Cells (x̄±s, n=3)
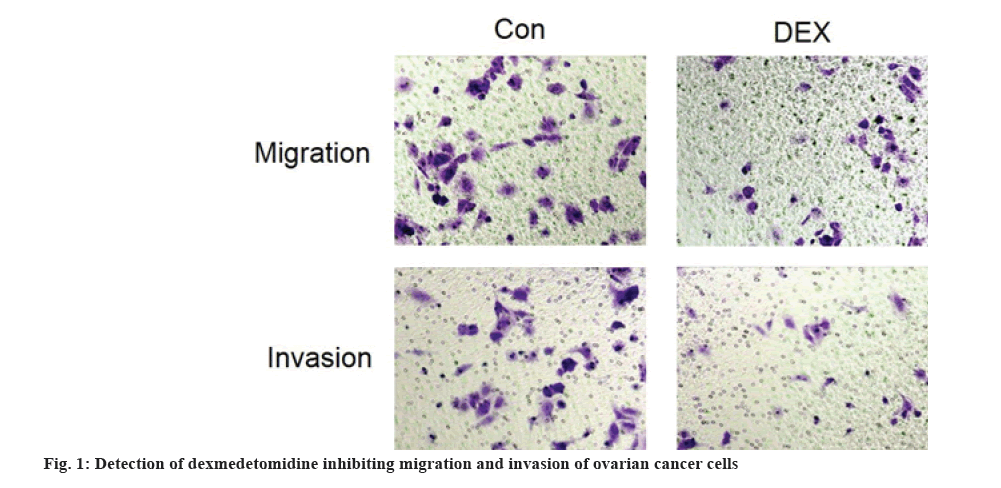
Results are as shown in fig. 1 and Table 2, the number of migration and invasion of SKOV3 cells were significantly decreased in the DEX group compared with the Con group (p<0.05).
| Group | Migration (number) | Invasion (number) |
|---|---|---|
| Con | 77.47±11.21 | 63.73±8.49 |
| DEX | 28.43±5.46* | 14.48±3.54* |
| t | 6.812 | 9.274 |
| p | 0.002 | 0.001 |
Note: *p<0.05 compared with the Con group
Table 2: DEX Inhibiting Migration and Invasion of Ovarian Cancer Cells (x̄±s, n=3)
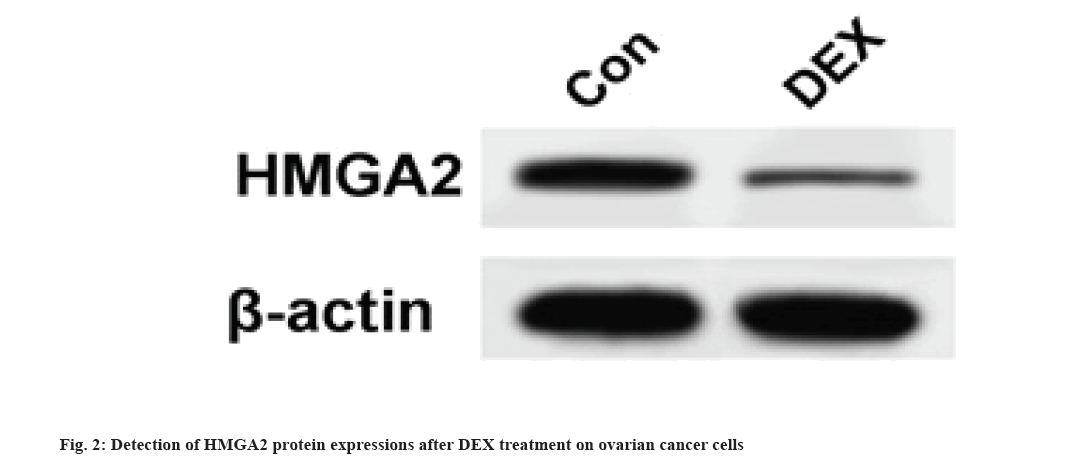
Results are as shown in fig. 2 and Table 3. Compared with the Con group, the DEX group exhibited significantly lower HMGA2 messenger RNA (mRNA) expression and higher HMGA2 protein expression in SKOV3 cells (fig. 2) (p<0.05).
| Group | HMGA2 mRNA | HMGA2 protein |
|---|---|---|
| Con | 1.01±0.07 | 0.78±0.09 |
| DEX | 0.38±0.04* | 0.28±0.03* |
| t | 13.535 | 9.129 |
| p | 0 | 0.001 |
Note: *p<0.05 compared with the Con group
Table 3: Effects of DEX on HMGA2 Expression in Ovarian Cancer Cells (x̄±s, n=3)
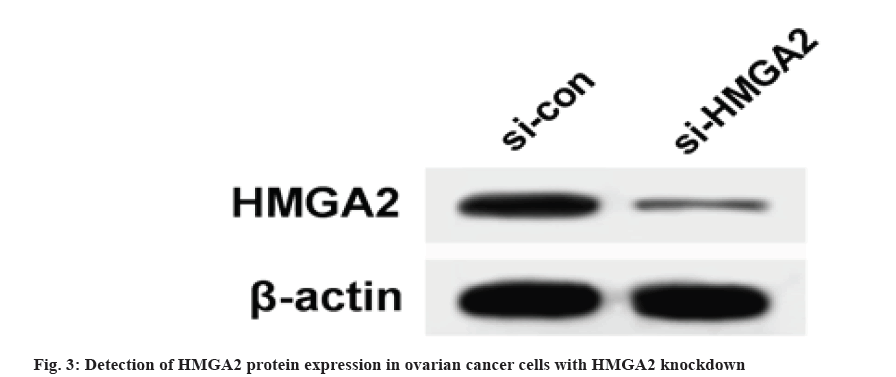
Results are as shown in fig. 3 and Table 4, the protein expression of HMGA2 in SKOV3 cells of si-HMGA2 group (0.21±0.04) was significantly lower than that of si-con group (0.72±0.08) and the number of cell proliferation, migration and invasion at 48 h and 72 h in si-HMGA2 group were lower than those in si-con group (p<0.05).
| Group | HMGA2 protein | OD 490 nm | Migration (number) | Invasion (number) | ||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||
| si-con | 0.72±0.08 | 0.44±0.05 | 0.67±0.08 | 1.19±0.11 | 93.58±10.72 | 77.83±6.49 |
| si-HMGA2 | 0.21±0.04* | 0.36±0.05 | 0.46±0.05* | 0.76±0.08* | 35.71±5.83* | 21.52±3.84* |
| t | 9.876 | 1.96 | 3.856 | 5.476 | 8.214 | 12.934 |
| p | 0.001 | 0.122 | 0.018 | 0.005 | 0.001 | 0 |
Note: *p<0.05 compared with si-con group
Table 4: Effects Of Low HMGA2 Expression on Proliferation, Migration and Invasion of Ovarian Cancer Cells (x̄±s, n=3)
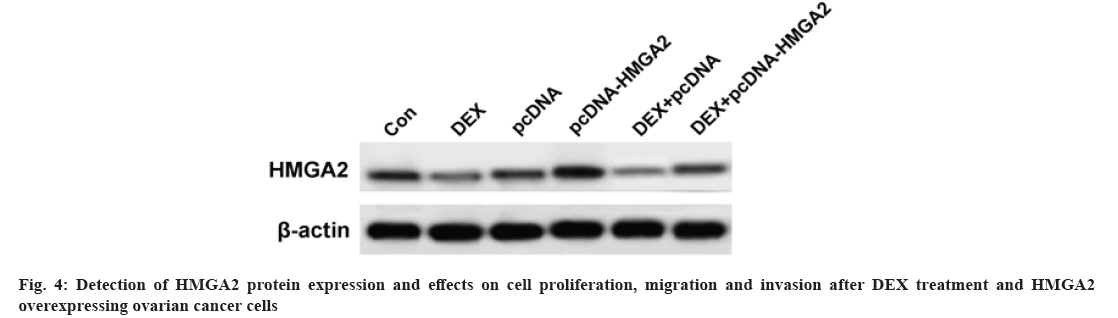
Results are as shown in fig. 4 and Table 5, the HMGA2 protein expression, cell proliferation, migration number and invasion number of SKOV3 cells at 48 h and 72 h were significantly decreased in the DEX group compared with the Con group; HMGA2 protein expression, cell proliferation, migration and invasion numbers in SKOV3 cells at 48 h and 72 h were significantly higher in the pcDNA-HMGA2 group compared with the pcDNA group; HMGA2 protein expression, cell proliferation, migration and invasion of SKOV3 cells at 48 h and 72 h were significantly higher in the DEX+pcDNA-HMGA2 group compared with the DEX+pcDNA group (p<0.05).
| Group | HMGA2 protein | OD 490 nm | Migration (number) | Invasion (number) | ||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||
| Con | 0.74±0.08 | 0.44±0.04 | 0.65±0.08 | 1.18±0.12 | 98.74±11.08 | 73.83±6.49 |
| DEX | 0.31±0.03* | 0.38±0.05 | 0.46±0.06* | 0.74±0.08* | 40.46±4.37* | 23.46±3.56* |
| pcDNA | 0.65±0.05 | 0.42±0.04 | 0.58±0.05 | 0.96±0.11 | 86.49±8.48 | 62.52±6.16 |
| pcDNA-HMGA2 | 0.87±0.07# | 0.46±0.05 | 0.76±0.06# | 1.34±0.09# | 156.68±11.59# | 108.71±8.42# |
| DEX+pcDNA | 0.25±0.03 | 0.36±0.04 | 0.43±0.06 | 0.71±0.09 | 33.84±5.19 | 20.18±3.37 |
| DEX+pcDNA-HMGA2 | 0.57±0.06🙳^Δ | 0.41±0.05 | 0.60±0.07🙳^Δ | 1.05±0.11🙳^Δ | 76.52±9.64🙳^Δ | 57.54±6.60🙳^Δ |
| F | 55.341 | 2.015 | 10.918 | 17.867 | 76.562 | 90.029 |
| p | 0 | 0.149 | 0 | 0 | 0 | 0 |
Note: *p<0.05 compared with Con group; #p<0.05 compared with pcDNA group; 🙳p<0.05 compared with DEX+pcDNA group; ^p<0.05 compared with DEX group and Δp<0.05 compared with pcDNA-HMGA2 group
Table 5: Overexpression of HMGA2 affects DEX Inhibition of Ovarian Cancer Cell Proliferation, Migration and Invasion (x̄±s, n=3)
Ovarian cancer is a high mortality disease, which seriously threatens women’s physical health[8]. The range of clinical applications of DEX is quite broad, including sedation, anesthesia, organ protection, antagonists and so on[9]. Recently, new functions of DEX have been continuously discovered, such as increasing the postoperative prognosis rate in cancer patients[10]. Studies conducted by Zhao et al.[11] and Liu et al.[12] elucidated that DEX increases the awakening rate and reduces the incidence of cognitive dysfunction in elderly ovarian cancer patients who have undergone surgical radical surgery. Cai et al.[13] established a rat model of ovarian cancer and found through DEX treatment that DEX can inhibit the growth of tumors, promote cancer cell apoptosis, up-regulate the content of Cluster of Differentiation (CD)4+ and CD8+ T cells and inhibit p38 Mitogen-Activated Protein Kinase (p38MAPK)/ Nuclear Factor-kappa B (NF-κB) signaling pathway, revealing that DEX could inhibit p38MAPK/NF-κB signaling pathway to enhance immune function in rats with ovarian cancer. Recently, Lian et al.,[14] examined the effect of DEX on the proliferation, migration, invasion and apoptosis of ovarian cancer cells and found that DEX can inhibit ovarian cancer cell proliferation in a concentration dependent manner, promote their apoptosis and down-regulate the migration and invasion ability of ovarian cancer cells, suggesting that DEX can inhibit the malignant behavior of ovarian cancer cells. In this study, MTT assay and transwell assay were performed to detect the proliferation, migration and invasion of SKOV3 in DEX treated ovarian cancer cells and the results were found to be consistent with previous studies, that is, the inhibition of SKOV3 proliferation, migration and invasion ability was inhibited by DEX; further detection found that DEX down-regulated HMGA2 expression in SKOV3 cells, which may be one of the functional mechanisms of DEX.
HMGA proteins are multifunctional, which are involved in many basic physiological processes of cells, including chromatin organization, cell cycle control, differentiation and cellular senescence, which are able to specifically interact with many transcription factors (NF-kB, Activating Transcription Factor 2 (ATF2)/Jun Proto-Oncogene (c-Jun), E74 Like ETS Transcription Factor 1 (Elf-1), Serum Response Factor (SRF), Nuclear Transcription Factor Y (NF- Y), PU-1 and retinoic acid receptor) to participate in the formation of specific multi protein enhance complexes, thus also known as structural transcription factors. Studies by Huang et al.[15] and Nie et al.[16] showed that high expression levels of HMGA2 were correlated with poor prognosis, metastasis and stage in a variety of cancers. In a study of ovarian cancer, Wu et al.[17] found that Stanniocalcin 2 (STC2) was directly regulated by HMGA2 at the transcriptional level and overexpression of STC2 directly enhanced cell migration and invasion and the expression of HMGA2 was significantly positively correlated with STC2 expression, suggesting that HMGA2 together with STC2 is involved in the malignant progression of ovarian cancer[18]. Malek et al. in ovarian cancer cells with HMGA2 knockdown found that ovarian cancer cell proliferation was inhibited, growth 1 phase arrest and apoptosis was increased, and it was effective in inhibiting tumor growth in nude mice. In this study, we found that the proliferation, migration, invasion of SKOV3 cells were inhibited after knocking down HMGA2, which coincided with the previous reports; in addition, after overexpression of HMGA2, the effects of DEX in inhibiting the proliferation, migration and invasion of ovarian cancer cells were reversed, which not only indicated that DEX could exert anti-ovarian cancer functions by down-regulating HMGA2, but conversely up-regulating HMGA2 could also reversely regulate the therapeutic effects of DEX on ovarian cancer, providing a more sufficient basis for the anti-cancer function of DEX. It has been shown that ATIP3a-HMGA2-ERK signaling pathway is involved in the resistance of cisplatin resistant ovarian cancer cells[19], thus it is speculated that the ERK signaling pathway may also be one of the mechanisms by which DEX down-regulates HMGA2 and then regulates the ERK signaling pathway.
In summary, DEX may exert anti-proliferation, anti- migration and anti-invasion effects on ovarian cancer cells SKOV3 by directly down-regulating HMGA2 in ovarian cancer cells SKOV3, which may lay the foundation for DEX to be used in the clinical treatment of ovarian cancer.
Conflict of interests:
The authors declared no conflict of interests.
References
- Small Jr W, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, et al. Cervical cancer: A global health crisis. Cancer 2017;123(13):2404-12.
[Crossref] [Google Scholar] [PubMed]
- Brodersen J, Siersma V, Thorsen H. Consequences of screening in cervical cancer: Development and dimensionality of a questionnaire. BMC Psychol 2018;6(1):39.
[Crossref] [Google Scholar] [PubMed]
- Xu W, Yu W. Advances in the clinical use of dexmedetomidine. Med Recapitulate 2017;23(9):1830-4.
- Hu X, He D, Lu S. Effects of dexmedetomidine on perioperative stress responsive hormones and postoperative cellular immunity in patients undergoing radical breast cancer surgery. Pract J Cancer 2015;30(5):669-72.
- Sgarra R, Pegoraro S, Ros G, Penzo C, Chiefari E, Foti D, et al. High Mobility Group A (HMGA) proteins: Molecular instigators of breast cancer onset and progression. Biochim Biophys Acta Rev Cancer 2018;1869(2):216-29.
[Crossref] [Google Scholar] [PubMed]
- Fedele M, Paciello O, de Biase D, Monaco M, Chiappetta G, Vitiello M, et al. HMGA2 cooperates with either p27kip1 deficiency or Cdk4R24C mutation in pituitary tumourogenesis. Cell Cycle 2018;17(5):580-8.
[Crossref] [Google Scholar] [PubMed]
- Miao JT, Gao JH, Chen YQ, Chen H, Meng HY, Lou G. LncRNA ANRIL affects the sensitivity of ovarian cancer to cisplatin via regulation of let-7a/HMGA2 axis. Biosci Rep 2019;39(7):BSR20182101.
[Crossref] [Google Scholar] [PubMed]
- Tian W, Liu S, Liu Xi. Effects of propofol on clone formation, invasion, EMT and miR-143 expression in SKOV3 ovarian cancer cells. J Zheng Univ 2019;54(4):607-10.
- Wang Y, Li S, Gao Y. Protective effect of dexmedetomidine on oxygen glucose deprivation injury in PC12 cells. J Zheng Univ 2019;54(4):611-4.
- Guo Y, Xu J, Ji Xu. Effects of dexmedetomidine on perioperative inflammation and lung function protection in patients undergoing curative resection for lung cancer. J Southern Med Univ 2017;37(12):1673-7.
- Zhao J, Zhang M, Liu C. The effect of dexmedetomidine on postoperative awakening and cognitive function in elderly patients undergoing laparoscopic radical ovarian cancer. Oncol Progress 2018;16(5):626-8.
- Liu T, Zhang S, Han X. Effects of dexmedetomidine on awakening and cognitive function after laparoscopic radical ovarian cancer surgery in elderly patients. Zhejiang Clin Med J 2015;17(3):346-8.
- Cai QH, Tang Y, Fan SH, Zhang ZF, Li H, Huang SQ, et al. In vivo effects of dexmedetomidine on immune function and tumor growth in rats with ovarian cancer through inhibiting the p38MAPK/NF-κB signaling pathway. Biomed Pharmacother 2017;95(1):1830-7.
[Crossref] [Google Scholar] [PubMed]
- Lian H, Jiang L. Effects of dexmedetomidine on relevant biological behaviors of ovarian cancer cells. Pract Pharm Clin Remed 2018;21(4):369-73.
- Huang B, Yang J, Cheng Q, Xu P, Wang J, Zhang Z, et al. Prognostic value of HMGA2 in human cancers: A meta-analysis based on literatures and TCGA datasets. Front Physiol 2018;9:776.
[Crossref] [Google Scholar] [PubMed]
- Nie D, Zhang L, Guo Q, Mao X. High mobility group protein A2 overexpression indicates poor prognosis for cancer patients: A meta-analysis. Oncotarget 2018;9(1):1237-47.
[Crossref] [Google Scholar] [PubMed]
- Wu J, Lai M, Shao C, Wang J, Wei JJ. STC2 overexpression mediated by HMGA2 is a biomarker for aggressiveness of high-grade serous ovarian cancer. Oncol Rep 2015;34(3):1494-502.
[Crossref] [Google Scholar] [PubMed]
- Xi YN, Xin XY, Ye HM. Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells. Asian Pac J Trop Med 2014;7(4):289-92.
[Crossref] [Google Scholar] [PubMed]
- Ping HU. Effects of ATIP3a-HMGA2-ERK signaling pathway on cisplatin resistance in epithelial ovarian cancer. J Hebei Med Univ 2018;38(10):1179.