- *Corresponding Author:
- H. Liu
Department of Pharmacy,
Changde Vocational and Technical College,
Changde,
Hunan Province 415000,
China
E-mail: Lhw359@163.co
| Date of Received | 18 September 2020 |
| Date of Revision | 10 August 2021 |
| Date of Acceptance | 01 April 2022 |
| Indian J Pharm Sci 2021;84(2):400-406 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze the protective effect and mechanism of beta-asarone from Acorus tatarinowii on neuron injury in rats with Parkinson's disease. Thirty five male Sprague Dawley rats were randomly selected to establish the rat model of Parkinson's disease. The rats were divided into 5 groups as sham operation group, model group, low dose beta-asarone group, medium dose beta-asarone group and high dose betaasarone group, with 7 rats in each group. Rats in the sham operation group and model group were injected with the same amount of normal saline, while rats in the low dose group, middle dose group and high dose group were injected with 10 mg/kg, 20 mg/kg and 40 mg/kg beta-asarone respectively. The behavior and the expression of tyrosine hydroxylase, alpha-synuclein, superoxide dismutase, catalase, glutathione peroxidase, interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha, B-cell lymphoma 2-associated X protein, B-cell lymphoma 2 and caspase-3 in brain tissue were detected level detection. Compared with the sham operation group, the number of autonomic activities, roll down time, forelimb activity time, the expression levels of alpha-synuclein, superoxide dismutase, catalase, glutathione peroxidase and B-cell lymphoma 2 were significantly decreased and the expression levels of tyrosine hydroxylase, interleukin-1 beta, tumor necrosis factor-alpha, nitric oxide, interleukin-6, B-cell lymphoma 2-associated X protein and caspase-3 were significantly increased in the model group (p<0.05) The expression levels of alphasynuclein, superoxide dismutase, catalase, glutathione peroxidase and B-cell lymphoma 2 were significantly decreased, while the expression levels of tyrosine hydroxylase, interleukin-1 beta, tumor necrosis factoralpha, nitric oxide, interleukin-6, B-cell lymphoma 2-associated X protein and caspase-3 were significantly increased (p<0.05). Low dose of beta-asarone may inhibit oxidative stress and inflammatory reaction, inhibit cell apoptosis, inhibit the decrease of tyrosine hydroxylase expression and the overexpression of alpha-synuclein, so as to play a certain neuroprotective role and reduce neuron damage.
Keywords
Acorus tatarinowii, beta-asarone, Parkinson's disease, neuron damage, bradykinesia
Parkinson's disease is a degenerative disease of the nervous system. Its main clinical features are movement disorders such as tremor, bradykinesia, abnormal posture and gait and non-motor disorders such as cognitive disorders, sleep disorders and olfactory disorders[1]. According to relevant statistics, the incidence of Parkinson's disease is about 0.3 % and the prevalence rate in elderly people over 70 y old is more than 2 %. And the condition will gradually worsen over time, until the loss of motor function, which will have a serious impact on the patient's quality of life[2]. The pathogenesis of Parkinson's disease is more complicated. Studies have found that the interaction of mitochondrial dysfunction, Lewy body deposition, immune inflammation, oxidative stress and apoptosis can all lead to the formation of Parkinson's[3]. At present, Parkinson's treatment mainly uses levodopa, dopamine receptor agonists and other drugs, but long term use will cause complications such as "switching phenomenon" or dyskinesia[4]. Therefore, finding other drugs with neuroprotective effects has become a clinically important issue. Traditional Chinese medicine has the advantages of multi-target effects and low side effects, which has gradually attracted people's attention. Beta-asarone (β-asarone) is one of the effective ingredients of Shichangpu. Studies have found that it plays an important role in the treatment of central nervous system diseases[5]. However, its mechanism of action is still unclear. The purpose of this study is to explore the effect of β-asarone, a component of Shichangpu, on reducing neuronal damage in Parkinson's disease rats and its related mechanisms.
Materials and Methods
Experimental animals:
Thirty five male Sprague Dawley (SD) rats were randomly selected [Zhejiang Weitong Lihua Laboratory Animal Technology Co., Ltd., production license SCXK (Zhe) 2020-0002, use license SYXK (Zhe) 2019-0003]. Body weight (40±10) g, age of 6 w, all rats are free to eat and drink in the laboratory temperature (23°±2°), humidity (56 %±12 %), 12 h d and night.
Main instruments and reagents:
Brain stereotaxic instrument (Beijing Jinuotai Technology Development Co., Ltd., model: JNT-DTY); inverted fluorescence microscope (Shanghai Wumo Optical Instrument Co., Ltd., model: WMF-3580); low temperature high-speed centrifuge (Shanghai Hetian Scientific Instrument Co., Ltd., model: TG18G); ultra low temperature refrigerator (Meiling Biomedical, model: YCDEL450); electronic balance (Shenyang Longteng Electronics Co., Ltd., model: JD-2); micro syringe pump (Shenzhen Nuoshen Technology Co., Ltd., model: NOTON-16); 6-hydroxydopamine (6-OHDA) (Dalian Meilun Biological Technology Co., Ltd., specification: 200 mg); blocked goat serum (Shanghai Hengfei Biotechnology Co., Ltd.); immunohistochemistry kit (Shanghai Jingke Chemical Technology Co., Ltd.); Bicinchoninic Acid (BCA) protein detection kit (Beijing Soleibao Technology Co., Ltd.).
Modeling and grouping:
Establish a rat model of Parkinson's: Anesthetize the rat, fix it on a stereotaxic device, prepare the skin and fully expose the skull and bregma. In the past, fontanelle was the origin to mark the coordinates of the lateral ventricle. A dental drill was used to drill a hole on the surface of the skull and then the tube was buried and the needle was accurately inserted into the substantia nigra dense part. Directional injection of 2μg/μl of 6-OHDA 5μL at a rate of 1μL/min. The needle was stopped for 5 minutes, then slowly withdrawn, and the rat scalp was sutured.
Division into groups: The rats were divided into sham operation group, model group, β-asarone low dose group, β-asarone middle dose group and β-asarone high dose group, with 7 rats in each group. Rats in the sham operation group and the model group were injected with the same amount of normal saline, the low dose β-asarone group, the middle-dose β-asarone group and the β-asarone. The high dose group was injected with 10 mg/kg, 20 mg/kg and 40 mg/kg β-asarone for a total of 7 d.
Observation indicators:
Behavioral testing: Using field experiments (making a transparent box of 35 cm×35 cm×20 cm in a quiet and dark environment, engraving a grid of 6 cm×6 cm at the bottom and adapting the rat to the environment for 5 min. Observe the number of moving grids and standing times of rats within 5 min and take the average of multiple measurements), roller experiment (place the rat on a rotating rod instrument with a radius of 3 cm and a rotation speed of 25 r/min, adapt to 3 times, with an interval of 60 s each time, to detect the time required for the rat to fall down), forelimb activity time (fix the hind limbs of the rat and lift it, fix one of the forelimbs and the rat adapts 3 times, with an interval of 60 s each time, to detect the time required for the movement of the rat's forelimbs and take the average of the measurements) the behavior of the rats is evaluated.
After the mouse behavior test, the rats were put to death; the brain tissues of the rats were taken, fixed with 4 % formaldehyde and routinely made 4 μm thick paraffin cuts. Immunohistochemical method was used to determine the expression levels of Tyrosine Hydroxylase (TH) and alpha-synuclein (α-syn) in the brain tissues of rats in each group. Routinely deparaffinize to water and use citrate buffer for antigen retrieval. Incubate with 3 % hydrogen peroxide for 30 min at room temperature to inactivate endogenous enzymes. 5 % Bovine Serum Albumin (BSA) at room temperature for 30 min. Add α-syn and TH antibody, overnight at 4°. Biotinylated goat anti-rabbit Immunoglobulin G (IgG) was incubated at room temperature for 40 min, Diaminobenzidine (DAB) developed color and the positive color was brown-yellow. Wash with phosphate buffer solution 3 times between each step. Lightly counter stained with hematoxylin, dehydrated, transparent, mounted and observed under optical microscope. Using a computer image processing system, 10 fields of view were randomly taken from each slice, and the average optical density of the positively stained cells was determined.
Determination of oxidative stress indicators: Take brain tissue, put it in a homogenizer, add an appropriate amount of lysis solution and place it on ice to grind the tissue until it is fully lysed. Centrifuge and take the supernatant. Strictly follow the determination of Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GSH-Px) assay kit methods. Set up a standard tube group, a measuring tube group and a blank control tube group. The components of the measuring tube are the tissue sample before the reaction and the tissue sample after the reaction for comparison. The absorbance value at 470 nm is measured by ultraviolet spectrophotometry.
Inflammatory factor level determination: The brain tissue inflammatory factors Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α) level. The operation was carried out strictly in accordance with the procedures of the operation kit.
Western blotting was used to determine the expression levels of B-cell lymphoma 2-associated X protein (BAX), B-cell lymphoma 2 (Bcl-2) and caspase-3 in the brain tissues of rats in each group. Take the brain tissue to fully lyse and homogenize, centrifuge, take the supernatant, prepare the BCA working solution, add it to each well and mix well and determine the total protein content obtained. After Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDSPAGE) electrophoresis, transfer membrane, block, add primary antibody, wash with Tris-Buffered Saline with Tween 20 (TBST) and shake at 4°. Add secondary antibody and rinsed Polyvinylidene Difluoride (PVDF) membrane for antibody incubation, drop Electrochemiluminescence (ECL) developer solution and use gel image processing system to analyze the band optical density value.
Statistical methods:
This study was approved by the medical ethics committee. According to the Shapiro-Wilk test, to test whether the data is normally distributed, the measurement data conforming to the normal distribution were compared between multiple groups by single factor and multiple samples, and the independent sample t test was used between the two groups. In this group of studies, Statistical Package for the Social Sciences (SPSS) 20.0 statistical software was used for statistical analysis and the statistical results were regarded as statistically significant at p<0.05.
Results and Discussion
Compared with the sham operation group, the number of autonomous activities in the model group was significantly reduced, the roller falling time was significantly shortened and the forelimb activity time was significantly prolonged (p<0.05). Compared with the model group, with the increase of the dose of β-asarone, the number of voluntary activities was significantly reduced, the roller falling time was significantly shortened and the forelimb activity time was significantly prolonged (p<0.05) as shown in Table 1.
| Group | Cases | Field experiment (times) | Roller experiment (s) | Forelimb activity time (s) |
|---|---|---|---|---|
| Mock surgical group | 7 | 78.52±10.23 | 176.26±52.19 | 3.58±0.72 |
| Model group | 7 | 14.76±3.12a | 33.58±7.86a | 87.96±17.33a |
| β-asarone low dose group | 7 | 48.55±3.58ab | 125.84±8.58ab | 18.46±2.84ab |
| β-asarone medium dose group | 7 | 37.87±2.41abc | 97.42±5.47abc | 34.69±5.13abc |
| β-asarone high dose group | 7 | 30.15±2.84abcd | 63.41±7.83abcd | 46.47±5.94abcd |
| F | 141.17 | 36.3 | 97.89 | |
| p | <0.001 | <0.001 | <0.001 |
Note: Compared with the sham operation group, ap<0.05; bp<0.05 compared with the model group; cp<0.05 compared with the low dose β-asarone group and dp<0.05 compared with the middle dose β-asarone group
Table 1: Behavioral Index Detection of rats in each group (x̄ ±s)
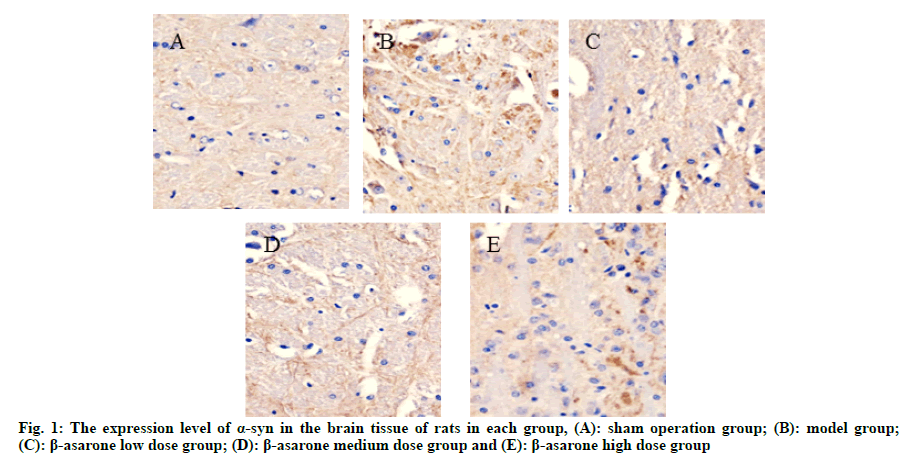
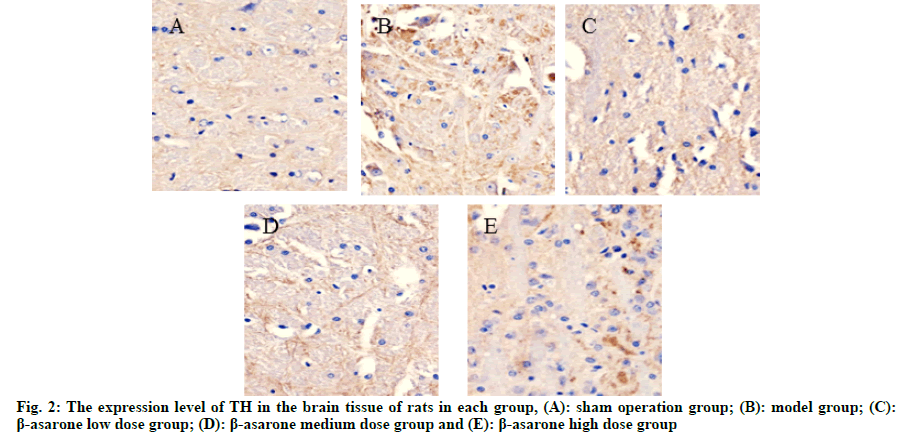
Compared with the sham operation group, the expression level of α-syn in the model group was significantly reduced and the expression level of TH was significantly increased (p<0.05); compared with the model group, with the increase of the dose of β-asarone, the expression level of α-syn decreased significantly and the expression level of TH increased significantly (p<0.05) as shown in fig. 1, fig. 2 and Table 2.
| Group | Cases | α-syn | TH |
|---|---|---|---|
| Mock surgical group | 7 | 118.29±11.74 | 901.25±86.57 |
| Model group | 7 | 224.16±17.84a | 176.58±22.16a |
| β-asarone low dose group | 7 | 140.16±7.33ab | 514.45±48.47ab |
| β-asarone middle dose group | 7 | 159.37±16.71abc | 408.69±41.71abc |
| β-asarone high dose group | 7 | 180.55±18.69abcd | 345.85±36.12abcd |
| F | 50.51 | 191.77 | |
| p | <0.001 | <0.001 |
Note: Compared with the sham operation group, ap<0.05; bp<0.05 compared with the model group; cp<0.05 compared with the low dose β-asarone group and dp<0.05 compared with the middle dose β-asarone group
Table 2: Expression Levels of a-syn and TH in the Brain Tissue of rats in each group (x̄ ±s)
Compared with the sham operation group, the expression levels of SOD, CAT and GSH-Px in the model group were significantly reduced (p<0.05). Compared with the model group, with the increase of β-asarone dose, the expression levels of SOD, CAT and GSH-Px were significantly reduced (p<0.05) as shown in Table 3.
| Group | Cases | SOD (U/mg) | CAT (U/mg) | GSH-Px (U/mg) |
|---|---|---|---|---|
| Mock surgical group | 7 | 14.42±2.11 | 189.43±4.17 | 78.54±4.02 |
| Model group | 7 | 4.43±1.68a | 63.68±2.87a | 21.59±2.74a |
| β-asarone low dose group | 7 | 12.29±1.19ab | 121.58±4.41ab | 53.69±3.58ab |
| β-asarone middle dose group | 7 | 9.93±1.25abc | 94.13±3.67abc | 35.07±3.04abc |
| β-asarone high dose group | 7 | 7.28±1.47abcd | 71.64±3.20abcd | 27.48±2.76abcd |
| F | 44.17 | 1309.51 | 350.88 | |
| p | <0.001 | <0.001 | <0.001 |
Note: Compared with the sham operation group, ap<0.05; bp<0.05 compared with the model group; cp<0.05 compared with the low dose β-asarone group and dp<0.05 compared with the middle dose β-asarone group
Table 3: Comparison of Oxidative Stress Indicators in the Brain Tissue of rats in each group (x̄ ±s)
Compared with the sham operation group, the expression levels of IL-1β, TNF-α, Nitric Oxide (NO) and IL-6 in the model group were significantly increased (p<0.05). Compared with the model group, as the dose of β-asarone increased, the expression levels of IL-1β, TNF-α, NO and IL-6 increased significantly (p<0.05) as shown in Table 4.
| Group | Cases | IL-1β (pg/mg) | TNF-α (pg/mg) | NO (μmol/g) | IL-6 (pg/mg) |
|---|---|---|---|---|---|
| Sham operation group | 7 | 1.13±0.24 | 84.29±4.35 | 4.86±0.25 | 1.48±0.38 |
| Model group | 7 | 5.97±0.52a | 324.28±7.97a | 13.17±1.74a | 4.74±0.47a |
| β-asarone low dose group | 7 | 3.12±0.35ab | 168.89±5.58ab | 6.22±0.83ab | 1.98±0.24ab |
| β-asarone middle dose group | 7 | 3.98±0.58abc | 219.47±5.64abc | 7.58±1.22abc | 2.68±0.48abc |
| β-asarone high dose group | 7 | 4.94±0.43abcd | 279.36±5.17abcd | 10.34±1.16abcd | 3.97±0.35abcd |
| F | 122.71 | 1795.32 | 58.84 | 83.66 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Compared with the sham operation group, ap<0.05; bp<0.05 compared with the model group; cp<0.05 compared with the low dose β-asarone group and dp<0.05 compared with the middle dose β-asarone Group
Table 4: Comparison of the Levels of Inflammatory Factors in the Brain Tissue of rats in each group (x̄ ±s)
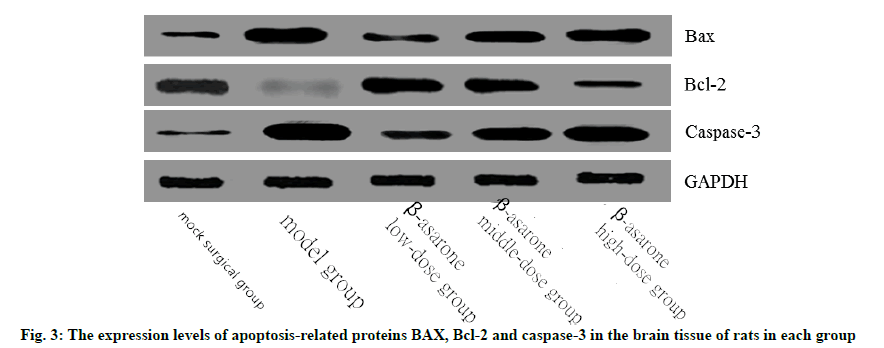
Compared with the sham operation group, the expression levels of BAX and caspase-3 in the model group increased significantly, while the expression levels of Bcl-2 decreased significantly (p<0.05). Compared with the model group, with the increase of the dose of β-asarone, the expression levels of BAX and caspase-3 increased significantly and the expression levels of Bcl-2 decreased significantly (p<0.05) as shown in fig. 3 and Table 5.
| Group | Cases | BAX | Bcl-2 | Caspase-3 |
|---|---|---|---|---|
| Sham operation group | 7 | 1.00±0.01 | 1.01±0.01 | 1.00±0.01 |
| Model group | 7 | 3.12±0.27a | 0.24±0.03a | 3.14±0.62a |
| β-asarone low dose group | 7 | 1.53±0.09ab | 0.82±0.12ab | 1.43±0.08ab |
| β-asarone middle dose group | 7 | 2.11±0.13abc | 0.71±0.04abc | 2.01±0.17abc |
| β-asarone high dose group | 7 | 2.73±0.26abcd | 0.44±0.07abcd | 2.55±0.30abcd |
| F | 157.05 | 149.48 | 50.18 | |
| p | <0.001 | <0.001 | <0.001 |
Note: Compared with the sham operation group, ap<0.05; bp<0.05 compared with the model group; cp<0.05 compared with the low dose β-asarone group and dp<0.05 compared with the middle dose β-asarone group
Table 5: The Expression Levels of Apoptosis-Related Proteins BAX, Bcl-2 and Caspase-3 in the Brain Tissue of Rats in each Group (x̄ ±s)
Parkinson's disease is the second largest degenerative disease of the central nervous system in the world after Alzheimer's disease. With the improvement of medical standards, the social population is gradually aging and the incidence of Parkinson's disease is increasing year by year and it has gradually become one of the most serious diseases threatening mankind. In recent years, traditional Chinese medicine has gradually been used in the treatment of Parkinson's disease. Studies have found that traditional Chinese medicine has the effects of anti-oxidant stress and protection of nerve cells. It can not only improve the clinical efficacy of Parkinson's disease, but also reduce the side effects of chemical drugs[6]. As a traditional Chinese medicine, Shichangpu has the effects of resuscitating and removing phlegm, rejuvenating the mind and strengthening the brain and is widely used in the treatment of stroke and senile dementia[7]. β-asarone, as an effective component of the traditional Chinese medicine Shichangpu, plays an important role in many central nervous system diseases such as epilepsy, senile dementia, cerebral ischemia and hypoxia[8]. This group of research takes SD rats as the research object, and establishes a Parkinsonian rat model induced by 6-OHDA. The aim was to observe the effect of β-asarone on neuronal damage induced by 6-OHDA in Parkinson's disease rats and its related mechanisms.
The main clinical manifestations of Parkinson's disease are movement disorders such as resting tremor, bradykinesia, abnormal posture and gait, and muscle rigidity. In this study, methods such as field experiment, roller experiment and forelimb exercise time were used to evaluate the behavioral changes of rats in each group. It was found that β-asarone can improve the dyskinesia of Parkinson's rats and the effect of low dose β-asarone is better.
The main pathological changes of Parkinson’s disease are degeneration of dopaminergic neurons in the substantia nigra and striatum, decreased dopamine transmitters in the striatum and the formation of lewy bodies in the remaining cells and α-syn is the main component of lewy bodies[9]. Studies have found that α-syn mutation or over expression can accelerate mitochondrial dysfunction, enhance sensitivity to oxidative stress and dopamine transporter-mediated toxicity, thereby promoting cell death[10]. TH is a ratelimiting enzyme of catecholamine active substances, which plays an important role in the synthesis of dopamine and its expression level is closely related to the occurrence and development of Parkinson's disease[11]. The results of this study found that β-asarone can significantly inhibit the decrease of TH expression and the overexpression of α-syn and the effect of low dose β-asarone is more obvious.
According to reports, oxidative stress is closely related to the occurrence and development of Parkinson's disease. Oxidative stress can significantly down-regulate the level of the midbrain antioxidant system, leading to the degeneration of substantia nigra dopaminergic neurons[12]. CAT and SOD are the main enzymes for scavenging oxygen free radicals. GSH-Px can protect the structure and function of cell membranes from the interference and damage of peroxides. Apoptosis is a kind of autonomous cell death, which plays an important role in the deformation and death of dopamine neurons in moles, and is also an important mechanism for the pathogenesis of Parkinson's disease[13]. Zhu et al.[14] found that the oxidative stress of dopaminergic neurons in Parkinson’s disease patients was significantly increased. Oxygen free radicals in the cell can enter the cell through a variety of receptor signals, leading to neuronal cell apoptosis. The results of this study found that β-asarone has obvious anti-oxidative stress and anti-apoptotic effects. In addition, some scholars believe that inflammation is involved in the occurrence of the disease[15]. When Parkinson’s disease occurs, neurons and endothelial cells are activated, and the levels of inflammatory factors such as TNF-α and IL- 1β are significantly increased, which promotes the release of other cytokines and forms a series of cascade reactions. Causes inflammation, damages neurons and promotes the occurrence of Parkinson's disease[16]. In this study, by detecting the expression levels of IL-1β, TNF-α, NO, IL-6 and other inflammatory factors in the brain tissue of each group of rats, it was found that β-asarone has obvious anti-inflammatory effects.
In summary, low dose β-asarone may inhibit oxidative stress and inflammatory response, inhibit cell apoptosis, inhibitthe decrease of TH expression and the overexpression of α-syn. So as to play a certain neuroprotective effect and reduce neuronal damage.
Acknowledgements
Hunan Natural Science Foundation Joint Fund Project (No. 2017JJ4011); General Research Project of Hunan Provincial Department of Education (No. 18C1226) and General Scientific Research Project of Changde Science and Technology Bureau (No. 2020S057).
Conflict of Interests
The authors declared no conflicts of interest.
References
- Liu M, Hu C, Zhang Y, Li Q, Zhang Q, Fang Y, et al. Effect of Huatan Jieyu granules in treatment of Parkinson's disease patients with sleep disorder identified as symptom pattern of phlegma-heat-stirring wind. J Tradit Chin Med 2020;40(3):461-6.
[Crossref] [Google Scholar] [PubMed]
- Hauser RA, Lew MF, Comella CL, Poewe W, Rascol O, Ferreira JJ, et al. Nighttime and morning OFF improvements with opicapone in patients with Parkinson's Disease and motor fluctuations: BIPARK-1 and BIPARK-2 pooled subanalyses. Parkinsonism Relat Disord 2020;79:e53-4.
- Riederer P, Müller T. Monoamine oxidase-B inhibitors in the treatment of Parkinson’s disease: Clinical–pharmacological aspects. J Neural Transm 2018;125(11):1751-7.
[Crossref] [Google Scholar] [PubMed]
- Goetz CG. New lessons from old drugs: Amantadine and Parkinson's disease. Neurology 1998;50(5):1211-2.
[Crossref] [Google Scholar] [PubMed]
- Yuan S, Liu J. Advances in the pharmacology of alpha-asarone. Jl Integr Tradit Chin West Med Cerebrovasc Dis 2020;18(8):67-9.
- Ning B, Zhang Q, Deng M. β-Asarone reduces the dose in Parkinson's disease model rats. J Guangzhou Univ Tradit Chin Med 2019; 36(6):889-96.
- Yang M, Yang N. Yang Nan's treatment of Parkinson's disease non-motor symptom drugs. J Chin Med 2020;271(12):121-4.
- Ning B, Zhang Q, Deng M. Bushen Kaiqiao recipe exerts neuroprotective effects on Parkinson's disease model rats by inhibiting the IRE1/XBP1 endoplasmic reticulum stress pathway. J Guangzhou Univ Tradit Chin Med 2019;36(4):549-55.
- Gupta V, Salim S, Hmila I, Vaikath NN, Sudhakaran IP, Ghanem SS, et al. Fibrillar form of α-synuclein-specific scFv antibody inhibits α-synuclein seeds induced aggregation and toxicity. Sci Rep 2020;10(1):1-4.
- Kumar ST, Donzelli S, Chiki A, Syed MM, Lashuel HA. A simple, versatile and robust centrifugation?based filtration protocol for the isolation and quantification of α?synuclein monomers, oligomers and fibrils: Towards improving experimental reproducibility in α?synuclein research. J Neurochem 2020;153(1):103-19.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Yang J, Wang X, Yao X, Han H, Gao Y, et al. Altered local field potential relationship between the parafascicular thalamic nucleus and dorsal striatum in hemiparkinsonian rats. Neurosci Bull 2019;35(2):315-24.
[Crossref] [Google Scholar] [PubMed]
- Dorszewska J, Kowalska M, Prendecki M, Piekut T, Koz?owska J, Kozubski W. Oxidative stress factors in Parkinson’s disease. Neural Regen Res 2021;16(7):1383-91.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Wang J, Zhang Q, Geng L, Yang Y, Wu N. Protective effect of fucoidan against MPP+-Induced SH-SY5Y cells apoptosis by affecting the PI3K/Akt pathway. Mar Drugs 2020;18(6):333.
[Crossref] [Google Scholar] [PubMed]
- Zhu J, Gao W, Shan X, Wang C, Wang H, Shao Z, et al. Apelin-36 mediates neuroprotective effects by regulating oxidative stress, autophagy and apoptosis in MPTP-induced Parkinson’s disease model mice. Brain Res 2020;1726:146493.
[Crossref] [Google Scholar] [PubMed]
- Brudek T. Inflammatory bowel diseases and Parkinson’s disease. J Parkinsons Dis 2019;9(s2):S331-44.
[Crossref] [Google Scholar] [PubMed]
- Ríos JP, Navarro CJ, Navarro MJ, Tapia MJ, Vera MJ, Arillo VC, et al. Association of Parkinson’s disease and treatment with aminosalicylates in inflammatory bowel disease: A cross-sectional study in a Spain drug dispensation records. BMJ open 2019;9(6):e025574.