- *Corresponding Author:
- D. Z. Hung
Department of Toxicology, China Medical University Hospital, Tanzi, Taichung 40447, Taiwan
E-mail: dzhung0224@gmail.com
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “95-101” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acute glufosinate ammonium poisoning can cause neurological complications and respiratory failure, which are usually delayed and difficult to predict. Serum ammonia level might be an indicator of severe glufosinate poisoning, but it has not been confirmed. We aimed to investigate the potential predictors of respiratory failure after glufosinate poisoning. We conducted a retrospective review of 21 cases of glufosinate poisoning between 2010 and 2019. Patients were assigned to intubated due to respiratory failure and non-intubated groups. The following characteristics were compared between these two groups; age, sex and period from poisoning to hospital arrival, vital signs, Glasgow coma scale, laboratory parameters and electrocardiogram measurements. Furthermore, the outcomes of morbidity and mortality were analyzed. Totally, 12 cases were be intubated and the other 9 were not intubated. Leukocytosis, hyperglycemia, increased serum creatinine, peak ammonia level and decreased Glasgow coma scale score found at emergency visits were significantly different between these two groups. The white blood cell count (median: 9100 vs. 15 785, p=0.046) and serum creatinine (median: 0.88 vs. 1.20, p=0.019) and blood sugar (median: 114.0 vs. 138.5, p=0.032) levels were higher and the initial Glasgow coma scale score was lower in the intubated group than in the non-intubated group (median: 15 vs. 13, p=0.030). Moreover, six patients who presented with a decreased Glasgow coma scale score and an increased ammonia level developed respiratory failure. In cases of glufosinate poisoning, leukocytosis, hyperglycemia, impaired renal function and decreased Glasgow coma scale score initially can use to predict respiratory failure. Moreover, conscious change combined with an early increase in serum ammonia level implied that, intensive monitoring should be required to prevent lethal complications from delayed onset of respiratory failure.
Keywords
Glufosinate ammonium, glutamine syntheses, neurotoxicity, hyperglycemia
Glufosinate Ammonium (GLA) is the main component of a broad-spectrum herbicide available in many countries and is widely used to control a wide range of weeds in agriculture[1,2]. Glufosinate is a glutamate analog and irreversibly inhibits Glutamine Syntheses (GS), which catalyzes glutamine from glutamate and ammonia. GS inhibition causes ammonia accumulation intracellular, resulting in photosynthesis inhibition, which causes tissue necrosis followed by plant death. Similar toxicity to the central nervous system was observed in mammals, which possibly occurred through the same inhibitory mechanism[3]. Acute GLA poisoning has various clinical manifestations, including circulatory collapse, neurotoxicity, fever, cardiac dysrhythmia, rhabdomyolysis and even death[4]. Although cardiovascular toxicity could result from formulated surfactant components rather than glufosinate itself in animals[5], neurotoxicity is mostly considered to be related to glufosinate. The neurotoxic manifestations of acute poisoning from GLA containing herbicides have been common and have included seizure, altered mental states, amnesia and central apnea, which require mechanical ventilation support and has a necessarily delayed onset after a latent period ranging from 4 h to 60 h after ingestion[6]. Except for refractory shock caused by severe poisoning, central apnea is considered to be the cause of death in GLA poisoning. The etiology of central apnea after a latent period is unclear and apnea sometimes occurs too quickly even before diagnosis, making it challenging for emergency physicians[7].
The early identification of those patients who will develop respiratory failure and require intensive care is difficult. The peak level of serum ammonia has been suggested to be a prognosis factor for severe GLA herbicide poisoning[1,8,9], but its role in predicting central apnea is unclear. Thus, in this study, we retrospectively analyzed case profiles and attempted to identify possible predictors that could help in the early identification of patients with GLA poisoning who would develop respiratory failure.
Materials and Methods
Study design and data:
In this study, adult patients with acute GLA from a single center between 2010 and 2019 were retrospectively reviewed. In total, 24 patients who presented to the Emergency Department of China Medical University Hospital with acute GLA poisoning were eligible for enrollment. Patients were excluded if more than 24 h had elapsed since GLA ingestion or if their data were incomplete. After these exclusion criteria were applied, 21 cases remained for analysis.
We divided the patients into two groups. Patients who developed respiratory failure and needed tracheal intubation were categorized into an intubated group. The others were assigned to the non-intubated group. The following characteristics were compared between these two groups; age, sex and period from poison ingestion to arrival at our hospital, vital signs, Glasgow Coma Scale (GCS) score, laboratory parameters and electrocardiogram measurements. Furthermore, initial ammonia concentration, peak ammonia concentration, shock index and the presence of Systemic Inflammatory Response Syndrome (SIRS) were measured.
The GCS was designed to assess the depth and duration of coma and impaired consciousness, based on ability to perform eye movements, speak and move their body. The scale helps to gauge the impact of a wide variety of conditions such as acute brain damage due to traumatic or vascular injuries or infections, metabolic disorders and even intoxication. The shock index was defined as the ratio of heart rate to systolic blood pressure.
SIRS is defined as being present if two or more of the following criteria are satisfied; body temperature >38° or <36°; heart rate >90 beats/ min; respiratory rate >20 breaths/min or Pressure of Carbon dioxide (PaCO2) <32 mmHg and White Blood Cell (WBC) count >12 000/mm3 or <4000/ mm3 or >10 % of bands form. Both shock index and positive SIRS criteria were reported by a study to be associated with the severity of respiratory complications in GLA poisoning[10]. Serum ammonia concentration was serially measured at least within 72 h and the peak concentration was defined as the highest value measured in this duration. Moreover, outcomes such as presence of aspiration pneumonia, seizure, conscious change, hypotension development, hospitalization days, days at the intensive care unit and mortality were compared between the two groups. This study was approved by the Ethics Review Board of China Medical University (CMUH109-REC1-117).
Statistical analysis:
Statistical analysis was performed using Statistical Analysis System (SAS) (version 9.4; SAS Institute, Inc., Cary, North Carolina). We use the Mann– Whitney U test and Chi-square (χ2) test to compare continuous and categorical data, respectively between the groups. The results are expressed in terms of median±range. The significance level was set at 0.05.
Results and Discussion
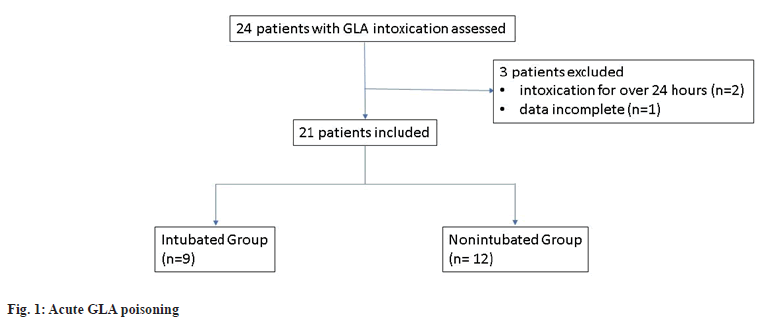
Between January 2010 and December 2019, 130 000- 170 000 patients visited our hospital’s emergency department annually. A total 24 consecutive patients with acute GLA poisoning were identified and 21 were enrolled after exclusion criteria were applied. Specifically, two patients were excluded because they arrived at our hospital after more than 24 h post ingestion and another patient was excluded because of a lack of data on serum ammonia concentration as shown in fig. 1.
Among our study participants, 14 (66.7 %) were men and the age range of participants was 26 y-88 y (mean age: 63 y). All patients had ingested GLA with suicidal intentions. Only six patients (28.6 %) called our center directly after intoxication. The remaining 15 patients (71.4 %) received first aid at a local hospital and were subsequently referred to our emergency department. The mean time between poisoning and arrival at our emergency department was 6.1 h (range: 1 h-24 h). No patient received extracorporeal removal therapy during admission. In total, 19 patients survived and 2 patients died after admission. Specifically, one patient died from severe pneumonia and the other patient died from severe gastrointestinal bleeding.
The intubated group comprised 12 patients (57.1 %). They were intubated for ventilator support due to acute respiratory failure. The mean time between poisoning and intubation was 9.2 h (range: 2 h-24 h). The mean days of ventilator support was 5 d, (range: 2.5 d-6.5 d). Patients in the non-intubated and intubated groups differed in terms of their initial GCS score (p=0.03), WBC count (p=0.046), serum creatinine (p=0.019), blood sugar (p=0.032), and peak serum ammonia (p=0.036). No significant differences were observed between the two groups in age, sex, duration between poisoning and arrival, initial vital signs, shock index, pH of blood gas, serum lactate, electrocardiogram QTc, or initial ammonia (Table 1). Furthermore, six patients with initial GCS score <13 along with increased ammonia levels developed respiratory failure and required ventilation support. Conversely, three patients who were lucid and had normal initial ammonia levels survived without developing respiratory failure.
| Variable | Non-intubated group median (IQR) n (%) | Intubated group median (IQR) n (%) | p value |
|---|---|---|---|
| Patient numbers | 9 | 12 | |
| Age (y) | 54.0 (45.0-67.0) | 68.5 (60.0-77.5) | 0.109 |
| Gender (%) | 0.349 | ||
| Male | 5 (55.5) | 9 (75.0) | |
| Interval between poisoning and ER arrival (h) | 3.0 (3.0-8.0) | 3.0 (2.0-5.0) | 0.277 |
| Vital signs when arrival | |||
| SBP | 139.0 (118.0-142.0) | 154.0 (126.5-166.0) | 0.064 |
| HR | 81.0 (77.0-90.0) | 95.5 (79.0-104.5) | 0.302 |
| BT | 36.5 (36.0-37.0) | 36.8 (36.2-37.4) | 0.591 |
| RR | 20.0 (19.0-21.0) | 19.0 (18.0-22.5) | 0.612 |
| GCS | 15.0 (15.0-15.0) | 13.0 (8.0-15.0) | 0.030 |
| Shock index | 0.65 (0.51-0.75) | 0.59 (0.55-0.73) | 0.87 |
| ABG | |||
| pH | 7.38 (7.37-7.42) | 7.30 (7.25-7.37) | 0.063 |
| Blood | |||
| WBC | 9100.0 (7100.0-11700.0) | 15 785.0 (10450.0-21350.0) | 0.046 |
| Hb | 13.6 (12.2-14.1) | 13.5 (11.4-15.7) | 0.971 |
| PLT | 275.0 (153.0-296.0) | 235.5 (209.5-295.5) | 0.859 |
| Neu | 81.1 (68.4-86.2) | 87.6 (76.4-91.3) | 0.213 |
| Lym | 9.9 (7.4-19.9) | 6.0 (4.5-13.6) | 0.135 |
| N/L | 9.0 (3.6-12.5) | 15.1 (5.22-20.13) | 0.302 |
| Cr | 0.88 (0.68-0.92) | 1.20 (0.89-2.61) | 0.019 |
| Sugar | 114.0 (94.0-126.0) | 138.5 (120.0-186.5) | 0.032 |
| Ammonia | 72.0 (52.0-88.0) | 145.5 (84.5-180.0) | 0.070 |
| Ammonia peak level | 114.0 (77.0-156.0) | 173.0 (139.0-356.5) | 0.036 |
| Lactate | 13.3 (8.5-17.7) | 20.7 (11.9-27.5) | 0.069 |
| EKG | |||
| QTc (ms) | 459.0 (407.0-464.0) | 441.0 (426.0-456.0) | 0.543 |
| SIRS =2 | 2 (22.2) | 7 (58.3) | 0.098 |
| Both initial GCS <13 and ammonia >70 (%) | 0 (0.0) | 6 (50.0) | 0.012 |
| Both initial GCS =14 and ammonia <70 (%) | 3 (33.3) | 0 (0.0) | 0.03 |
Table 1: Demographic Characteristics
Complications during hospitalization after GLA poisoning were seizure (9 patients, 42.9 %), shock (7 patients, 33.3 %) and pneumonia (9 patients, 42.9 %). Patients in the intubated group were more likely to develop pneumonia than were those in the nonintubated group (p=0.01; Table 2). Of the 12 patients in the intubated group, 11 patients (91.6 %) had a change in consciousness and one patient without such a change developed respiratory failure because of severe aspiration pneumonia.
| Complications | Non-intubated group median (IQR) n (%) | Intubated group median (IQR) n (%) | p value |
|---|---|---|---|
| Seizure (%) | 2 (22.2) | 7 (58.3) | 0.098 |
| Development of hypotension (%) | 1 (11.1) | 6 (50) | 0.061 |
| Pneumonia (%) | 1 (11.1) | 8 (66.7) | 0.010 |
| Outcome | |||
| ICU days | 1.5 (1.0-2.5) | 6.0 (5.0-8.5) | 0.001 |
| Total hospital days | 5.0 (3.0-11.0) | 11.0 (8.5-17.5) | 0.074 |
| Mortality (%) | 0 (0) | 2 (16.7) | 0.197 |
Table 2: Comparisons of Complications and Outcomes between the Two Groups
Glufosinate belongs to the class of organic phosphorus herbicide but does not inhibit acetylcholinesterase. GLA herbicide toxicity is mostly attributed to glufosinate itself and its surfactant. Glufosinate toxicity is usually neurological and surfactant toxicity is cardiovascular. The structural similarity of glufosinate and glutamate implicates the glutamatergic system as a target for glufosinate neurotoxicity[11]. However, the mechanism of delayed respiratory failure after poisoning remains unknown. A delay implies that more time is needed for the absorption of GLA or for its metabolism to a toxin to affect the respiratory center. Hori et al.[6] reported the presence of glufosinate in the spinal fluid of a patient developing complications from glufosinate poisoning with serious but 26 h delayed respiratory depression. They suggested that the delayed central nervous symptoms were related to the small amount of glufosinate that had entered the blood from the brain. The other most likely cause is central apnea because respiratory failure is often accompanied by changes in consciousness. Furthermore, direct toxicity to the lung or heart might play an additional role because few patients developed respiratory failure before a change in their mental state. Some subunits of N-methyl-d-aspartate receptors were identified in the lungs and airways of experimental animals. In vivo studies have shown that exogenous administration of high concentrations of glutamate or glutamate agonist can induce pulmonary edema and airway constriction[12]. Pesticide aspiration, pulmonary edema after heart and circulatory failure related to the surfactant, or lung inflammatory reaction caused by glufosinate could possibly explain these manifestations[13].
In our cases, the presence of leukocytosis and hyperglycemia increases in serum creatinine and peak ammonia level and a decrease in initial GCS score were associated with respiratory failure after GLA poisoning. Although the mechanism through which GLA suppresses the human central nervous system is still unknown, an altered level of consciousness is a well-known manifestation of GLA poisoning. Changes in consciousness has been reported in 21 %-72 % of patients with GLA poisoning during hospitalization[1,8,14]. Moreover, 2/3 of our patients eventually developed a change in consciousness. In most cases, consciousness change was delayed. Patients who ingested large doses presented with rapid onset of impaired GCS initially and were positively associated with severe neurotoxicity[14].
WBC count often increased in cases of acute poisoning and it might be due primarily to acute stress and secondarily to hypostatic pneumonia following a prolonged coma[15]. Furthermore, leukocytosis is a useful tool for estimating the prognosis of acute poisoning in some cases. Kumar et al.[16] found that the leukocyte count on admission can be a prognostic marker in patients with organophosphate poisoning. Furthermore, leukocytosis was noted in severe poisoning from glyphosate, also an organic phosphorus herbicide, with significant differences in the WBC counts between survivors and nonsurvivors[17,18]. Similar with data from other studies on organ phosphorus poisoning, our data show that the WBC count was significantly higher in the intubated group than in the non-intubated group for GLA poisoning.
Hyperglycemia is a less common complication of poisoning than hypoglycemia is, but it has been reported after methanol poisoning[19], aluminum phosphide poisoning[20] and theophylline overdose. Additionally, the relationship between organophosphate poisoning and hyperglycemia has been frequently discussed. Panda et al.[21] reported that blood sugar level might be a predictor of hospital morbidity and mortality in acute organophosphate poisoning. Moon et al.[22] reported that fatality risk independently increased in OP-poisoned patients without diabetes because their venous glucose level was high. A proposed mechanism of poisoning-related hyperglycemia is stress-induced hyperglycemia, which often occurs in critically ill patients and is activated by the release of catecholamine’s, glucagon and growth hormones[23]. The other mechanisms that are frequently associated with poisoning are pancreas damage[24] and insulin resistance[25]. In GLA poisoning, stress may lead to hyperglycemia because studies have yet to indicate that GLA exerts a toxic effect on the pancreas. Thus, further investigations are required.
The intubated group exhibited poor renal function. Furthermore, Lee et al.[8] reported that 22.2 % of the patients with GLA poisoning developed self-limited acute kidney injury. The etiology was unclear. In pesticide poisoning, renal toxicity was sometimes attributed to the surfactant or solvent. Significantly high serum creatinine was found in deceased patients with poisoning of glyphosate and its surfactant[26]. The anionic surfactant of GLA is a well-known toxin, which affects the cardiovascular system[5] and is cytotoxic[27]. We cannot rule out its role in renal toxicity and further study is required. Furthermore, renal function impairment at presentation might indicate that the patient had chronic kidney disease before poisoning. Glufosinate is usually excreted unchanged from the kidney[28]; thus, renal function impairment may delay toxin excretion and worsen the outcome. Hence, patients with high serum creatinine must be closely observed.
Ammonia is a famous neurotoxin. GS in the liver is a crucial enzyme for ammonia removal in addition to the urea cycle[29]. Based on the glufosinate poisoning mechanism, the serum ammonia level might increase when GS activity is irreversibly inhibited in acutely poisoned patients. Several studies have attempted to use serum ammonia to predict neurotoxicity in GLA poisoning. In a study by Inoue et al.[10] ammonia level was not associated with severe toxicity in GLA poisoning. Several studies have reported that initial ammonia level, peak ammonia level and serial ammonia trend are associated with neurological complications[1,8,9]. Unlike these studies, we used delayed respiratory failure as an outcome measurement because we believe it is crucial in clinical monitoring and is closely related to mortality. Our results demonstrate no significant difference in terms of initial serum ammonia level between the two groups (p=0.07), but the results might be different if more cases had been analyzed. Therefore, our results require validation in future studies.
Because both ammonia and glutamate are neurotoxins, we chose to analyze serum ammonia level and the level of consciousness, which are the most classic manifestations of glufosinate poisoning. Six patients who presented with decreased GCS scores and increased serum ammonia level initially developed respiratory failure and required ventilator support later. Conversely, three patients with normal ammonia levels and clear consciousness initially all did not develop respiratory failure. Although we had a limited sample size, our results are valuable. Thus, we should pay attention to patients of acute GLA poisoning with disturbed consciousness and increased ammonia level in the emergency room. Patients with normal GCS and ammonia level are less likely to develop respiratory failure.
Inoue et al.[10] reported that two patients with positive SIRS criteria presented with severe toxicity, including respiratory failure, seizure and severe consciousness disturbance. SIRS is useful in evaluating sepsis severity. Furthermore, it is used for predicting the prognosis of trauma and gastrointestinal surgery[30,31]. However, our results indicated no difference between the two groups in terms of SIRS criteria.
Moon et al. reported a high neurotoxicity risk in patients with increasing follow-up ammonia levels and >101 μg/dl peak serum ammonia. Furthermore, they noted that the time of peak ammonia level was usually 12 h-24 h after admission, similar to the latent period of 4 h-60 h for severe complications, such as seizure, respiratory failure and change in consciousness, reported in the literature[6]. Moreover, our results showed that the mean period between poisoning and tracheal intubation was 9.2 h. Thus, physicians in a crowded emergency department must vigilantly observe GLA poisoning patients. According to our findings, to prevent complications, airway protection should be performed in patients with GLA poisoning presenting with changes in consciousness and increased serum ammonia.
Our study has several limitations. First, our sample size was small because patients from only one medical center were studied. Second, ours was a retrospective study; thus, some information might be lost and results might deviate. For example, the coingestion of ethanol or other medications, GLA formulation and amount of poison ingested may not always be documented correctly. Third, we did not measure the serum concentration of glufosinate to discover its clinical contributions, although doing so mightnot be practicalin an emergency setting.
Leukocytosis, hyperglycemia, impaired renal function, and decreased GCS scores on admission can use to predict respiratory failure in patients with acute glufosinate poisoning. Moreover, we should pay attention to patients with a simultaneous presentation of unconsciousness and hyperammonemia.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cha YS, Kim H, Lee Y, Choi EH, Kim HI, Kim OH, et al. The relationship between serum ammonia level and neurologic complications in patients with acute glufosinate ammonium poisoning: A prospective observational study. Hum Exp Toxicol 2018;37(6):571-9.
[Crossref] [Google Scholar] [PubMed]
- Watanabe T, Sano T. Neurological effects of glufosinate poisoning with a brief review. Hum Exp Toxicol 1998;17(1):35-9.
[Crossref] [Google Scholar] [PubMed]
- Hack R, Ebert E, Ehling G, Leist KH. Glufosinate ammonium—some aspects of its mode of action in mammals. Food Chem Toxicol 1994;32(5):461-70.
[Crossref] [Google Scholar] [PubMed]
- Roberts DM. Herbicides. Goldfrank's Toxicologic Emergencies. 11th ed; 2019. p. 1478.
- Koyama K, Koyama K, Goto K. Cardiovascular effects of a herbicide containing glufosinate and a surfactant: In vitro and in vivo analyses in rats. Toxicol Appl Pharmacol 1997;145(2):409-14.
[Crossref] [Google Scholar] [PubMed]
- Hori Y, Tanaka T, Fujisawa M, Shimada K. Toxicokinetics of DL-glufosinate enantiomer in human BASTA poisoning. Biol Pharm Bull 2003;26(4):540-3.
[Crossref] [Google Scholar] [PubMed]
- Lee JH, Kim YW. Prognostic factor determination mortality of acute glufosinate-poisoned patients. Hum Exp Toxicol 2019;38(1):129-35.
[Crossref] [Google Scholar] [PubMed]
- Lee DK, Youk H, Kim H, Kim OH, Go J, Kim TH, et al. Initial serum ammonia as a predictor of neurologic complications in patients with acute glufosinate poisoning. Yonsei Med J 2016;57(1):254-9.
[Crossref] [Google Scholar] [PubMed]
- Moon JM, Chun BJ. Serial ammonia measurement in patients poisoned with glufosinate ammonium herbicide. Hum Exp Toxicol 2016;35(5):554-61.
[Crossref] [Google Scholar] [PubMed]
- Inoue Y, Onodera M, Fujita Y, Fujino Y, Kikuchi S, Endo S. Factors associated with severe effects following acute glufosinate poisoning. Clin Toxicol 2013;51(9):846-9.
[Crossref] [Google Scholar] [PubMed]
- Lantz SR, Mack CM, Wallace K, Key EF, Shafer TJ, Casida JE. Glufosinate binds N-methyl-D-aspartate receptors and increases neuronal network activity in vitro. Neurotoxicology 2014;45:38-47.
[Crossref] [Google Scholar] [PubMed]
- Sami IS, Gill S, Pulido O editors. Glutamate toxicity in lung and airway disease. Glutamate receptors in peripheral tissue: Excitatory transmission outside the CNS: Springer, Boston, MA; 2005. p. 191-6.
- Maillet I, Perche O, Pâris A, Richard O, Gombault A, Herzine A, et al. Glufosinate aerogenic exposure induces glutamate and IL-1 receptor dependent lung inflammation. Clin Sci 2016;130(21):1939-54.
[Crossref] [Google Scholar] [PubMed]
- Mao YC, Hung DZ, Wu ML, Tsai WJ, Wang LM, Ger J, et al. Acute human glufosinate-containing herbicide poisoning. Clin Toxicol 2012;50(5):396-402.
[Crossref] [Google Scholar] [PubMed]
- Lämmermann T, Germain RN. The multiple faces of leukocyte interstitial migration. Semin Immunopathol 2014;36(2):227-51.
[Crossref] [Google Scholar] [PubMed]
- Kumar S, Agrawal S, Raisinghani N, Khan S. Leukocyte count: A reliable marker for the severity of organophosphate intoxication? J Lab Physicians 2018;10(02):185-8.
[Crossref] [Google Scholar] [PubMed]
- Kim YH, Lee JH, Hong CK, Cho KW, Park YH, Kim YW, et al. Heart rate–corrected QT interval predicts mortality in glyphosate-surfactant herbicide–poisoned patients. Am J Emerg Med 2014;32(3):203-7.
[Crossref] [Google Scholar] [PubMed]
- Lee HL, Chen KW, Chi CH, Huang JJ, Tsai LM. Clinical presentations and prognostic factors of a glyphosate—surfactant herbicide intoxication a review of 131 cases. Acad Emerg Med 2000;7(8):906-10.
[Crossref] [Google Scholar] [PubMed]
- Sanaei-Zadeh H, KazemiEsfeh S, Zamani N, Jamshidi F, Shadnia S. Hyperglycemia is a strong prognostic factor of lethality in methanol poisoning. J Med Toxicol 2011;7(3):189-94.
[Crossref] [Google Scholar] [PubMed]
- Mehrpour O, Alfred S, Shadnia S, Keyler DE, Soltaninejad K, Chalaki N, et al. Hyperglycemia in acute aluminum phosphide poisoning as a potential prognostic factor. Hum Exp Toxicol 2008;27(7):591-5.
[Crossref] [Google Scholar] [PubMed]
- Panda S, Nanda R, Mangaraj M, Rathod KP, Mishra PK. Glycemic status in organophosphorus poisoning. J Nepal Health Res Council 2015;13(31):214-9.
[Google Scholar] [PubMed]
- Moon JM, Chun BJ, Cho YS. Hyperglycemia at presentation is associated with in hospital mortality in non-diabetic patient with organophosphate poisoning. Clin Toxicol 2016;54(3):252-8.
[Crossref] [Google Scholar] [PubMed]
- McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 2001;17(1):107-24.
- Ikizceli I, Yurumez Y, Avsaroğullari L, Kucuk C, Sozuer EM, Soyuer I, et al. Effect of interleukin-10 on pancreatic damage caused by organophosphate poisoning. Reg Toxicol Pharmacol 2005;42(3):260-4.
[Crossref] [Google Scholar] [PubMed]
- Lasram MM, Dhouib IB, Annabi A, El Fazaa S, Gharbi N. A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 2014;322:1-3.
[Crossref] [Google Scholar] [PubMed]
- Lee CH, Shih CP, Hsu KH, Hung DZ, Lin CC. The early prognostic factors of glyphosate-surfactant intoxication. Am J Emerg Med 2008;26(3):275-81.
[Crossref] [Google Scholar] [PubMed]
- Song HY, Kim YH, Seok SJ, Gil HW, Yang JO, Lee EY, et al. Cellular toxicity of surfactants used as herbicide additives. J Korean Med Sci 2012;27(1):3-9.
[Crossref] [Google Scholar] [PubMed]
- Hirose Y, Kobayashi M, Koyama K, Kohda Y, Tanaka T, Honda H, et al. A toxicokinetic analysis in a patient with acute glufosinate poisoning. Hum Exp Toxicol 1999;18(5):305-8.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Eid T, Hassel B, Danbolt NC. Novel aspects of glutamine synthetase in ammonia homeostasis. Neuro chem Int 2020;140:104809.
[Crossref] [Google Scholar] [PubMed]
- Napolitano LM, Ferrer T, McCarter Jr RJ, Scalea TM. Systemic inflammatory response syndrome score at admission independently predicts mortality and length of stay in trauma patients. J Trauma Acute Care Surg 2000;49(4):647-53.
[Crossref] [Google Scholar] [PubMed]
- Haga Y, Beppu T, Doi K, Nozawa F, Mugita N, Ikei S, et al. Systemic inflammatory response syndrome and organ dysfunction following gastrointestinal surgery. Crit Care Med 1997;25(12):1994-2000.
[Crossref] [Google Scholar] [PubMed]