- *Corresponding Author:
- N. Mohamed Shah
Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
E-mail: noraida_mshah@ukm.edu.my
| Date of Submission | 31 December 2018 |
| Date of Revision | 17 May 2019 |
| Date of Acceptance | 11 August 2019 |
| Indian J Pharm Sci 2019;81(5):913-921 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Neonates in the intensive care unit diagnosed with suspected early onset neonatal sepsis are common. Empiric antibiotic therapy is crucial for the management of early onset neonatal sepsis. The purpose of this study was to describe the empiric antibiotic de-escalation practice in suspected early onset neonatal sepsis and evaluate its outcome within 7 days of birth. This was a single centre study conducted at a Malaysian tertiary hospital where new-borns were admitted to the hospital within 72 h of birth and prescribed with an empiric antibiotic therapy were included. Relevant data were retrieved from the patients’ electronic medical records. A total of 529 new-borns were screened and 278 (mean gestational age- 34.9±3.73 weeks, birth weight- 2.34±0.87 kg) were included. Common maternal risk factors of early onset neonatal sepsis identified were prolonged rupture of membranes (>18 h), positive culture of the maternal high vaginal swab and meconium-stained amniotic fluid. Majority of patients presented with respiratory symptoms and empiric antibiotics, namely crystalline penicillin and gentamicin were started within 24 h of birth. The early empirical antibiotic de-escalation was practiced in 98.5 % of patients; of these, 59 % had aminoglycoside withdrawal after the second dose and continuation of crystalline penicillin as a monotherapy (de-escalation group I). The remaining patients had complete antibiotic withdrawal prior to 72 h of exposure (de-escalation group II). New-borns in the de-escalation group I had significantly longer treatment duration and poor Apgar scores at 1 min of life (Apgar score ≤3; p<0.05). In addition, 6 patients had positive blood cultures and 2.2 % of patients were classified as treatment failure. Hence, an early antibiotic de-escalation may potentially be practiced in suspected early onset neonatal sepsis.
Keywords
Early onset neonatal sepsis, empiric antibiotics, antibiotic duration, de-escalation
Antibiotics such as ampicillin, gentamicin, cefotaxime, and vancomycin are listed among top 10 medications that are commonly used in neonatal intensive care units (NICUs)[1]. In suspected early onset neonatal sepsis (EONS), ampicillin together with gentamicin or cefotaxime is used empirically, as per recommendations by American Academy of Pediatrics (AAP) and South Australian Perinatal guidelines[2,3]. One study conducted at a university hospital in Malaysia has reported that 84.3 % of new-borns at the NICU are prescribed with crystalline penicillin (C-penicillin) and gentamicin as a first-line therapy[4]. It is in concordance with the Malaysian guidelines, where C-penicillin and gentamicin combination is recommended in suspected EONS[5,6].
New-borns exposed to maternal risk factors or those who develop early clinical manifestations are commonly prescribed with empiric antibiotics in the NICU[2,7]. The World Health Organisation (WHO) has prepared a guideline on possible risks and clinical manifestations that require an early antibiotic coverage to reduce risks of mortality and morbidity related to neonatal sepsis, as well as has emphasized on the importance of starting empiric antibiotics[8]. It should be started as early as possible because a delay in neonatal sepsis treatment can increase the risk of new-born mortality, especially if caused by Gram-negative organisms[9,10].
It is a big challenge for paediatricians to prescribe empiric antibiotics, especially in certain difficult scenarios where organisms rarely grow in the blood culture possibly due to single sampling and small blood volume[1,11,12]. Hence, the treatment duration of an empiric antibiotic remains controversial[2]. Most of established guidelines have recommended reviewing empiric antibiotic treatment outcomes after 36 h of exposure and a substantial amount of evidence has reported that shorter treatment duration in suspected EONS does not interfere with desirable outcomes[13,14].
Early empiric antibiotic de-escalation is not common in neonatal sepsis; however, it can possibly be done in critically ill patients[15]. Gentamicin is usually used as a synergistic to penicillin, and it also eradicates Gram-negative organisms[16]. The WHO guideline has suggested at least 2 doses of intramuscular gentamicin and 2 d of intravenous ampicillin as antibiotic prophylaxis in new-borns with risks of sepsis[8]. Therapeutic outcomes need to be monitored if more than 2 doses of gentamicin are given[17]. A previous study conducted at the Malaysian government hospitals has shown that the most common microorganism isolated from EONS patients is Gram-positive with a good sensitivity against first-line antibiotics[11]. Hence, withdrawal of gentamicin after the second dose can be done particularly in patients where probabilities of sepsis are low and new-borns waiting for laboratory confirmation remain well during 72 h of life. It may potentially be implemented as an early de-escalation strategy in suspected EONS patients to minimize unnecessary antibiotic exposure and hazards of blood collection. This investigation is aimed to describe the empiric antibiotic de-escalation practice in suspected EONS patients admitted to the NICU and evaluate its outcome within 7 d of birth.
Materials and Methods
Study design:
This study was a prospective observational study conducted at the NICU of a tertiary hospital in Malaysia. The NICU has 44 beds, managed by a neonatologist. It also has full-time clinical pharmacists who actively participate in the management of medical therapies including antibiotics for all patients. No intervention or interruption to management activities was done by the researcher during the study period.
Inclusion and exclusion criteria:
Patients were eligible to be included if they were admitted to the NICU with the diagnosis of suspected EONS and started on empiric antibiotics within 72 h of birth. Patients who received empiric antibiotic treatments after 72 h of birth were excluded. No active intervention was done during study period. Ethical approval was obtained from the Medical Research and Ethics Committee of the Ministry of Health Malaysia (NMRR-17-1882-36914 (IIR)).
Data collection:
Records of all patients admitted to the NICU specialist hospital within 72 h of life, in a period between September 1, 2017 and December 31, 2017, were prospectively screened. The data of eligible patients were collected from patients’ electronic medical records and manual prescriptions. The data included patients’ demographic profiles, risk factors, clinical manifestations, prescribed antibiotics, types of organisms, de-escalation practices, and treatment outcomes up to 7 d of birth. Microbiological analyses included blood cultures prior to the commencement of empiric antibiotics.
Patients were diagnosed with suspected EONS when exposed to risk factors or showed early signs of an infection within 72 h of birth[2,7]. Risk factors included maternal fever, chorioamnionitis, prolonged rupture of membrane (PROM; >18 h), positive maternal group B Streptococcus (GBS) status, incomplete intrapartum antibiotic prophylaxis (IAP), meconium stained amniotic fluid (MSAF), premature or low birth weight, and perinatal asphyxia[2,7,8,18].
Early signs of an infection observed included fever, cardiovascular, respiratory, and metabolic instabilities, gastrointestinal symptoms such as food intolerance, vomiting, and abdominal distension, and seizure[8,18]. Reported laboratory examinations included preantibiotic blood culture, complete blood count including platelet and WBC count, and C-reactive protein (CRP) level[19,20].
Early empiric antibiotic de-escalation is defined as reduction of the antibiotic spectrum by reducing the number of antibiotics (de-escalation group I) or discontinuation of all antibiotics in absence of any obvious infection within 72 h of treatment (de-escalation group II)[21,22]. Non-de-escalation is defined as continuation of empiric antibiotics even after having negative culture results and escalation is defined as switching first-line antibiotics to broader-spectrum antibiotics[15] due to deteriorating conditions observed via clinical assessments or laboratory confirmations within 72 h of therapy. Classification of patients into de-escalation I, de-escalation II or non-de-escalation groups was made by the paediatrician in charge.
Primary endpoint was expressed by treatment failure and suspicion of second infection within 7 d of birth. Treatment failure was defined as mortality within 7 d of birth with one of the causes of mortality being related to sepsis or the need for empiric antibiotic escalation within 72 h of therapy due to no improvement or deterioration of patient’s conditions[12]. A second infection within 7 d of birth was defined as suspicion of recurrent infection via clinical assessments or laboratory confirmations[12].
Data analysis:
The data analysis was carried out using IBM® SPSS® for Windows version 23. Frequencies and percentages of each continuous variable were calculated and presented in table formats. For practice-related differences, categorical variables were assessed using Pearson Chi-Square test (χ2 test) or Fisher’s exact test and continuous variables were assessed using Mann Whitney U test where the median were compared between the groups. For all statistical analyses, the significance was set at 0.05.
Results and Discussion
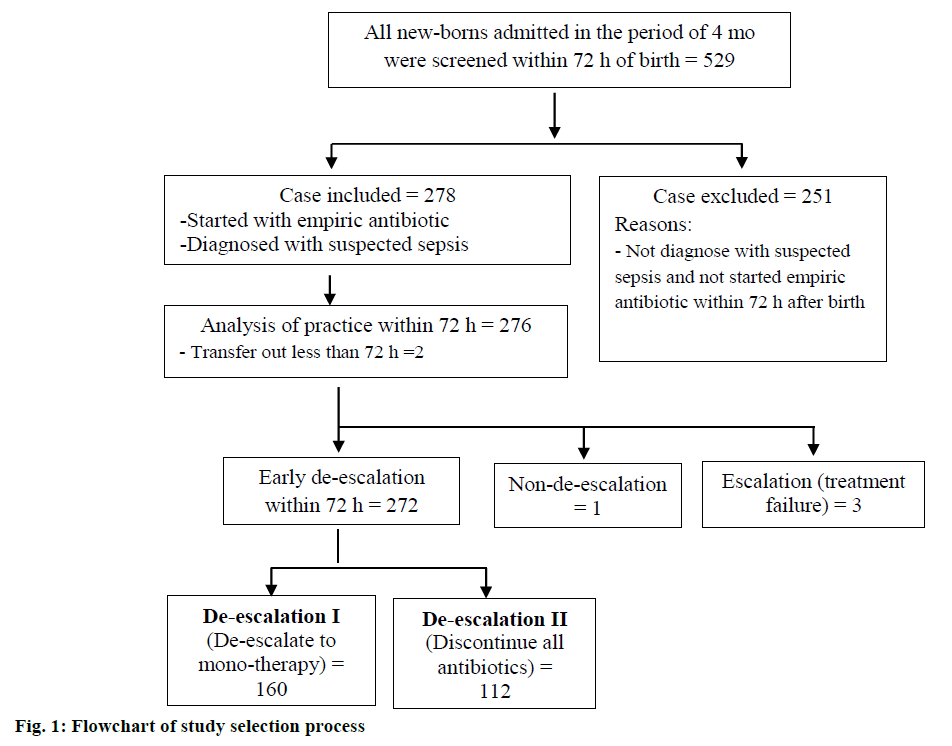
A total of 529 new-borns were admitted to the NICU within 72 h of birth, over a period of 4 mo. All patients were screened for inclusion and exclusion criteria and 278 (52.5 %) patients were eligible to be included in the study. Two patients were transferred to another hospital for further surgical management; thus, 276 patients were enrolled for further practice-related analyses (fig. 1).
Early empiric antibiotic de-escalation was practiced in 272 (98.5 %) patients; subsequently, these patients were prospectively stratified into 2 groups according to practices, de-escalation group I (n=160) and de-escalation group II (n=112). Only one case was continued with empiric antibiotics after 72 h of treatment as the patient underwent spinal surgery; in addition, 3 patients required antibiotic escalation within 72 h of birth (fig.1).
The mean gestational age was 34.9±3.734 w and birth weight was 2.34±0.873 kg. Table 1 describes demographics and risk factors of the 2 groups. Both groups had no significant difference in terms of maternal characteristics, maternal risk factors, and neonatal characteristics (p>0.05). The only significant difference between the groups was higher Apgar score ≤3 at 1 min of life in the de-escalation group I. A total of 125 (46 %) patients were admitted up to 7 d of birth to NICU and the rest stay longer than a week (Table 1). Out of 125 patients, 118 (94 %) were discharged from NICU, 1 (0.8 %) transferred to other facility and 6 (4.8 %) patients died within 7 d of birth.
| Characteristics | De-escalation I (n=160) | De-escalation II (n=112) | p-value |
|---|---|---|---|
| Maternal | |||
| Age (y), mean±SD | 29.84±5.840 | 29.95±5.118 | 0.837 |
| Antepartum antibiotic exposure, n (%) | 15 (9.4) | 12 (10.7) | 0.716 |
| Intrapartum antibiotic prophylaxis, n (%) | 32 (20) | 21 (18.8) | 0.798 |
| IAP completed >4 h prior delivery, n (%) | 22 (13.8) | 18 (16.1) | 0.160 |
| Lack of antenatal care, n (%) | 25 (15.6) | 9 (8) | 0.063 |
| Maternal risk | |||
| PROM > 18 hours, n (%) | 22 (13.8) | 21 (18.6) | 0.290 |
| Maternal pyrexia >38°C, n (%) | 7 (4.4) | 3 (2.7) | 0.341 |
| Maternal high vaginal swab/ urine culture positive, n (%) | 19 (11.9) | 13 (11.5) | 0.911 |
| History of GBS carrier, n (%) | 3 (1.9) | 0 (0) | 0.198 |
| Meconium stained amniotic fluid/foul smelling liquor, n (%) | 23 (14.5) | 11 (9.7) | 0.207 |
| Chorioamnionitis, n (%) | 2 (1.3) | 3 (2.7) | 0.652 |
| Perinatal asphyxia, n (%) | 3 (1.9) | 1 (0.9) | 0.804 |
| Neonatal | |||
| Gestational age (w), mean±SD | 34.81±3.741 | 35.13±3.612 | 0.543 |
| Premature (<36 w), n (%) | 94 (58.8) | 66 (58.9) | |
| Term (≥37 w), n (%) | 66 (41.3) | 46 (41.1) | |
| Birth weight (mean±SD kg) | 2.325±0.886 | 2.40±0.837 | 0.391 |
| Gender | |||
| Male, n (%) | 96 (60) | 66 (58.9) | |
| Female, n (%) | 64 (40) | 46 (41.1) | |
| Race | |||
| Malay, n (%) | 121 (75.6) | 83 (74.1) | 0.776 |
| Chinese, n (%) | 6 (3.8) | 7 (6.3) | |
| Indian, n (%) | 16 (10) | 12 (10.7) | |
| Others, n (%) | 17 (10.6) | 10 (8.9) | |
| Length of stay (d), n (%) | |||
| ≤ 7 | 68 (42.5) | 57 (50.9) | |
| 8-28 | 60 (37.5) | 35 (31.3) | |
| >28 | 32 (20.0) | 20 (17.9) | |
| Neonatal risk | |||
| Ventilation support, n (%) | 0.771 | ||
| Intubated, n (%) | 28 (17.5) | 18 (16.1) | |
| Not intubated, n (%) | 87 (54.4) | 58 (51.8) | |
| Surfactant, n (%) | 7 (4.4) | 6 (5.4) | 0.709 |
| APGAR score @ 1 min, mean±SD | 7.19±2.486 | 7.63±1.992 | 0.280 |
| Score ≤3, n (%) | 18 (11.3) | 5 (4.5) | 0.048* |
| Apgar score @ 5 min, mean±SD | 8.91±2.058) | 9.18±1.651 | 0.525 |
| Score ≤3, n (%) | 5 (3.1) | 3 (2.7) | 0.567 |
De-escalation I- withdrawal one of the antibiotics; De-escalation II- withdrawal of all antibiotics; IAP- intrapartum antibiotic prophylaxis; PROM- prolong rupture of membrane; GBS- group B streptococcus; COH- head circumference and APGAR- appearance, pulse, grimace, activity, respiration; *statistically significant at p<0.05
Table 1: Study populations demographics and risk factors
Table 2 shows the summary of empiric antibiotic treatment. In general, most of the empiric treatments were started with the C-penicillin and gentamicin combination within 24 h of birth. The treatment duration was significantly longer in the de-escalation group I as compared to the de-escalation group II.
| Treatment summary | De-escalation I (n=160) | De-escalation II (n=112) | p-value | ||
|---|---|---|---|---|---|
| First dose empiric antibiotic, n (%) | 0.051 | ||||
| Within 24 h of birth | 150 (93.8) | 108 (96.4) | |||
| Within 48 h of birth | 9 (5.6) | 1 (0.9) | |||
| Within 72 h of birth | 1 (0.6) | 3 (2.7) | |||
| Initial antibiotic combinations, n (%) | |||||
| C-penicillin+gentamicin | 153 (95.6) | 109 (97.3) | |||
| C-penicillin+amikacin | 5 (3.1) | 3 (2.7) | |||
| Ampicillin+gentamicin | 2 (1.3) | 0 (0.0) | |||
| Treatment duration (d), mean±SD* | 3.944±1.096 | 2.263±0.251 | 0.005* | ||
De-escalation I- withdrawal one of the antibiotics; De-escalation II- withdrawal all antibiotics; C-penicillin- crystalline penicillin. *Statistically significant at p<0.05
Table 2: Pattern of empirical antibiotics used for early onset neonatal sepsis
Clinical manifestations observed in suspected EONS are listed in Table 3. For early clinical manifestations, there was no significant difference between the 2 groups (p>0.05). Respiratory symptoms showed highest clinical manifestation nearly 60 % in both groups and mostly included recession (42 %), followed by nasal flaring (29 %), tachypnea (24 %), grunting (21 %), and cyanosis (6 %). Besides respiratory symptoms, more than 30 % of new-borns were presented with acidosis confirmed by blood pH. Gastrointestinal symptoms were present in less than 10 % of patients; in addition, less than 5 % of patients were presented with thermoregulatory, cardiac, or hypotension symptoms.
| Early clinical manifestations, n (%) | De-escalation I (n=160) | De-escalation II (n=112) | p-value | |
|---|---|---|---|---|
| Thermoregulatory symptoms | 6 (3.8) | 6 (5.4) | 0.525 | |
| Fever | 1 (0.6) | 5 (4.4) | 0.094 | |
| Hypothermia | 5 (3.1) | 2 (1.8) | 0.704 | |
| Cardiac symptoms | 6 (3.8) | 5 (4.4) | 0.769 | |
| Tachycardia | 2 (1.3) | 2 (1.8) | 1.000 | |
| Bradycardia | 4 (2.5) | 3 (2.7) | 1.000 | |
| Hypotension | 3 (1.9) | 1 (0.9) | 0.646 | |
| Respiratory symptoms | 93 (58.1) | 67 (59.8) | 0.780 | |
| (Cyanosis, grunting, recession tachypnea, nasal flaring) | ||||
| Gastrointestinal symptoms | 11 (6.9) | 9 (8) | 0.464 | |
| (Feeding intolerance, vomiting) | ||||
| Metabolic symptoms | 67 (41.9) | 41 (36.3) | 0.462 | |
| Acidosis | 58 (36) | 38 (33.9) | 0.693 | |
| Hypoglycemia | 11 (6.9) | 3 (2.7) | 0.166 | |
| Seizure | 2 (1.3) | 2 (1.8) | 1.000 | |
De-escalation I- withdrawal of one of the antibiotics; De-escalation II- withdrawal of all antibiotics
Table 3: Early clinical manifestations of study population
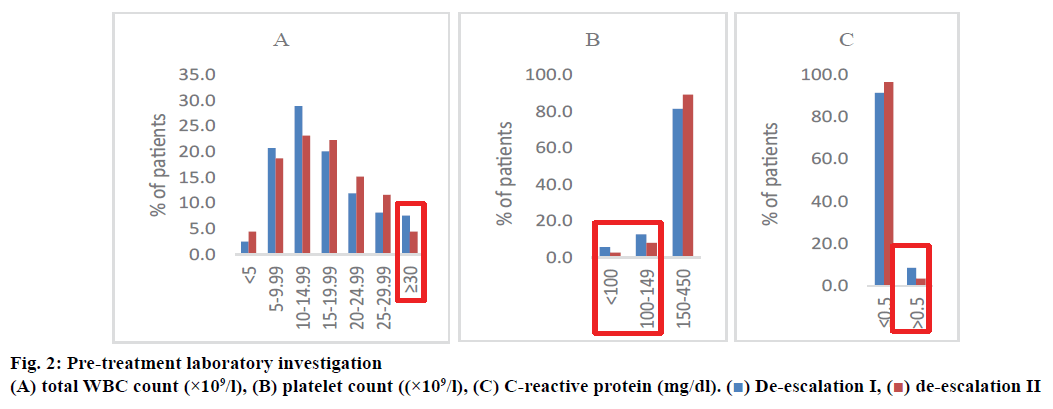
Fig. 2 presents the pre-treatment laboratory investigation. Number of patients with total WBC count analysed were 159 patients, platelet count 157 patients, and CRP 82 patients in the deescalation group I. For the de-escalation group II, the corresponding number of patients was 112, 112, and 60, respectively. The difference in these parameters was not significant between the 2 groups (p>0.05). However, the de-escalation group I showed slightly higher percent of total WBC count >30×109/l (3 %), platelet count <150×109/l (3 %), and CRP >0.5 mg/dl (5.2 %) compared to the de-escalation group II.
Table 4 shows information on pathogens that were isolated from blood cultures prepared prior to the empiric antibiotic administration. A total of 6 organisms were isolated from the de-escalation group I. Five pathogens were Gram-positive, and one was Gram-negative. GBS was isolated from a case of confirmed sepsis. Other isolated pathogens were labelled as contaminant sample in the pathology report. The antibiotic sensitivity was tested only for Sphingomonas (pseudo.) paucimobilis, which was reported to have sensitivity toward gentamicin. Repeated blood culture was done right after notification of positive preliminary result of first blood culture. All repeated blood cultures were no growth and none of the patients were under the treatment failure category.
| Case | GA/BW | Pathogens | EONS risk factors | Reason for EONS evaluation | Treatment description |
|---|---|---|---|---|---|
| 1 | 32/1.63 | Bacillus sp. | • Premature with low birth weight | Feeding intolerance | Gentamicin 2 doses C-penicillin 5 d |
| 2 | 36/2.23 | Bacillus sp. | • Premature with low birth weight | Hypoglycaemia | Gentamicin 2 doses C-penicillin 5 d |
| 3 | 39/3.16 | Cellulomonas sp. | • PROM >18 h | Well-appearing new born, evaluated because of risk factors | Gentamicin 2 doses C-penicillin 5 d |
| • GBS positive with adequate IAP | |||||
| 4 | 39/2.9 | Group B streptococcus | • Meconium stained amniotic fluid | Respiratory distress at birth with tachycardia | Gentamicin 2 doses C-penicillin 10 d |
| 5 | 37/3.87 | Staphylococcus, coagulase negative | None | Respiratory distress at birth | Gentamicin 2 doses C-penicillin 5 d |
| 6 | 37/3.24 | Sphingomonas (pseudo.) paucimobilis | None | Respiratory distress at birth | Gentamicin 2 doses C-penicillin 7 |
GA- gestational age; BW- birth weight; EONS- early onset neonatal sepsis; C-penicillin- crystalline penicillin; PROM- prolong rupture of membrane; IAP- intrapartum antibiotic prophylaxis; GBS- group B streptococcus
Table 4: Pathogens isolated during the study period
In this study, treatment failure was observed in 6 patients (2.2 %), which included 3 escalation patients and 4 mortality patients, including the one from escalation patients, who died within 7 d of birth due to sepsis. Three other mortality patients with additional prematurity-related complications were from the deescalation group I. The difference in the number of second infection within 7 d of birth was not significant between the 2 groups, and it occurred between d 5 to d 6 of birth (Table 5).
| Treatment outcome up to 7 d of birth | Early de-escalation | Escalation (n=3) | p-value | ||
|---|---|---|---|---|---|
| De-escalation I (n=160) | De-escalation II (n=112) | ||||
| Antibiotic escalation within 72 h due to deteriorating condition, n/deatha | - | - | 3/1 | ||
| Death within 7 d of birth due to sepsis, n (%)a | 3 (1.9)b | 0 (0.0) | 1b | ||
| Second infection within 7 d of birth, n (%) | 14 (8.8) | 11 (9.8) | 0 (0) | ||
| days of life, mean±SD) | 6.07±0.616 | 5.36±0.924 | 0.151 | ||
De-escalation I- withdrawal of one of the antibiotics; de-escalation II- withdrawal of all antibiotics, atreatment failure; b4 mortality patients were complicated with underlying extreme and very preterm issue (corrected gestational age= 24-32 weeks)
Table 5: Primary endpoint, outcome at 7 D of birth
In more than half of new-born admissions, empiric antibiotics were started within 24 to 72 h of birth. In this study, the majority were boys, premature with low birth weight, which are common characteristics of suspected EONS, as reported in previous studies[2,18,23]. PROM (>18 h), positive culture of maternal high vaginal swab (HVS), and MSAF were the main risk factors found in this study; in addition, respiratory symptoms showed highest clinical manifestation in both the groups. All these findings are similar to previous studies[18,23].
According to AAP and National Institute for Health and Care Excellence (NICE) guidelines, PROM (>18 h) and positive culture of maternal HVS were considered as risk factors that require empiric antibiotic treatments[2,17]. MSAF was not considered as a criterion to start empiric antibiotics. It usually occurs in 13 % of patients, which is similar to our finding; in addition, at least 5 % of these patients are presented with meconium aspiration syndrome (MAS)[24,25]. Empiric antibiotics in MAS are questionable according to a randomised control trial from India, which has not found any difference in the incidence of infection between new-borns with MAS who are with or without empiric antibiotic treatments[26].
In general, neonatal sepsis is a leading cause of mortality and morbidity, especially in EONS, and the presumptive antibiotic use is very important and needs to be started without delay[27]. Although early initiation of empiric antibiotic could potentially save lives, there are limited data on the antibiotic treatment duration[28]. This is a big concern as it can lead to antibiotic resistance and increase treatment costs[29].
Existing guidelines have suggested reviewing the outcome of empiric antibiotics between 36 and 48 h after the treatment[2,17]. However, prolonged use of empiric antibiotics in EONS is still an issue because of the lack of sensitive and specific diagnostic tests for detecting sepsis[30] and lower amount of positive blood cultures in new-borns[11]. Early empiric antibiotic de-escalation can be one of the options to reduce unnecessary antibiotic use[21], especially in asymptomatic and clinically well new-borns[2].
From the global perspective, GBS is the most common organism causing EONS[31]. The Centres for Disease Control and Prevention guidelines have recommended using IAP as a preventive measure, which has reduced the number of proven GBS patients markedly[31]. In developing countries, widespread use of IAP has caused changing of causative organisms from predominant Gram-positive to Gram-negative[32,33]. A recent Malaysian study has shown that nearly 80 % of patients are exposed to Gram-positive organisms, despite the IAP exposure[11]. Our study showed that 19-20 % of patients were exposed to IAP and only 14-16 % completed IAP>4 h prior delivery, which may give less impact on changing the causative organism.
There were 2 types of de-escalation practices identified in this study; withdrawal of gentamicin after the second dose and continuation of C-penicillin as a monotherapy and withdrawal of all antibiotics[21,22] within 72 h of treatment. Early empiric antibiotic de-escalation was common in this centre with a de-escalation rate of 98.5 %. The rate is reported to be lower in a retrospective study conducted in Ohio, USA, where only 40 % of early de-escalation was practiced in new-borns with negative blood cultures[12].
Early withdrawal of gentamicin may be an ideal approach in situations where Gram-positive organisms are the predominant one to cause EONS[11] in clinically well neonates waiting for the laboratory confirmation. This approach is also recommended in a guideline by the French National Agency for Accreditation and Evaluation in Health on neonatal sepsis[14]. In this guideline, 2 doses of aminoglycoside are recommended in clinically well new-borns except for those with meningitis and Gram-negative bacillipositive cultures[14].
In this study, there was no significant difference in terms of maternal risks and clinical manifestations between the 2 types of de-escalation practices. However, significantly prolonged C-penicillin monotherapy (de-escalation group I) was observed. According to the guideline on managing EONS, treatment duration can be determined by blood culture reports, WBC and platelet counts, and CRP level[2]. It justifies the prolonged use of antibiotics in the de-escalation group I where high percentages of WBC count >30×109/l, platelet count <150×109/l, CRP >0.5 mg/dl and positive blood cultures were noticed. Besides, the de-escalation group I also had significantly poor Apgar score ≤3 at 1-min of life.
There are studies on the impact of early de-escalation of antibiotics in EONS. One randomised control trial conducted in Iran has compared the early de-escalation of all antibiotics at d 3 of the treatment with d 5 of the treatment in suspected EONS[23]. The findings have shown that there is no difference in treatment failure, defined as reappearance of sepsis symptoms within 2 w of the treatment discontinuation, between the 2 groups.
Another study conducted in Ohio, USA, has compared between the antibiotic duration of ≤3 d and ≥7 d, wherein neonates with negative blood cultures in the ≤3 d group are discontinued with the antibiotic[13]. They have concluded that the clinical outcome is not compromised, and the survival rate remains the same even with shorter treatment duration[13]. However, no evidence was found on the impact of gentamicin withdrawal after the second dose in our study.
In this study, the treatment failure rate characterized by antibiotic escalation within 72 h and mortality within 7 d due to sepsis was 2.2 %. This rate is far lower than the one reported by a study conducted in Estonia, wherein the failure rate assessed using the same criteria is 14 %[12]. Our finding proved that an early de-escalation practice in EONS did not increase the treatment failure rate more than that observed in the published data[12]. However, this was a single centre study with clinician preference practice; thus, it is better to compare between the de-escalation practice and the non-de-escalation practice to obtain more robust research findings especially mortality outcome. Overall study results showed that an early empiric antibiotic de-escalation practice in suspected EONS did not increase the incidence of treatment failure. Hence, an early antibiotic de-escalation may potentially provide desirable clinical outcomes with minimum antibiotic exposure.
Acknowledgements:
Authors would like to thank the Director-General of Health, Malaysia, for the permission to publish this paper. Authors are grateful to the Head of Paediatric Department, Sungai Buloh Hospital, for the support towards this research project. Authors wish to thank the Pharmacy Department and the Neonatal Intensive Care Unit of Sungai Buloh Hospital for technical support.
Conflicts of interest:
There are no conflicts of interest.
References
- Clark RH, Bloom BT, Spitzer AR, Dale R. Reported medication use in the Neonatal Intensive Care Unit: Data from a large national data set. Pediatrics 2006;117(6):1979-87.
- Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis guidance for the clinician in rendering. Pediatric care. Pediatrics 2012;129(5):1006-15.
- South Australian Perinatal Practice Guideline: Neonatal sepsis. Government of South Australia; 2014. Available from: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/perinatal/perinatal+practice+guidelines.
- Awaisu A, Sulaimaa SAS, Ibrahim MIM, Saad A. Antimicrobials utilization and outcomes of neonatal sepsis among patients admitted to a University Teaching Hospital in Malaysia. Eastern J Med 2007;12:6-14.
- Pediatric Protocol. 3rd ed. Malaysia: Ministry of Health; 2012. Available from: https://mpaeds.my/paediatric-protocols-for-malaysian-hospitals-3rd-edition-2012/.
- National Antibiotic Guideline. 2nd ed. Malaysia: Ministry of Health; 2014. Available from: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/national-antibiotic-guideline-2014-full-versionjun2015_1.pdf.
- Jefferies AL. Management of term infants at increased risk for early-onset bacterial sepsis; Canadian Paediatric Society, Fetus and Newborn Committee, Ottawa, Ontario. Paediatr Child Health 2017:22(4):223-8.
- WHO. Antibiotic use for sepsis in neonates and children: 2016 Evidence Update; 2016. Available from: https://www.who.int/selection_medicines/committees/expert/21/applications/s6_paed_antibiotics_appendix4_sepsis.pdf
- Ghotaslou R, Ghorashi Z, Nahaei M-R. Klebsiella pneumoniae in neonatal sepsis: A 3-year-study in the Pediatric Hospital of Tabriz, Iran. Jpn J Infect Dis 2007;60:126-8.
- Benjamin DK, DeLong E, Cotton CM, Garges P, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: Increased with gram-negative bacteremia and candidemia, but not gram-positive bacteremia. J Perinatol 2004;24:175-80.
- Ibrahim NA, Manan MM. Early onset neonatal sepsis pathogens in Malaysian Hospitals: Determining empiric antibiotic. Int J Pharmacol Pharm Sci 2014;8(10):686-90.
- Metsvaht T, Ilmoja M-L, Parm Ü, Maipuu L, Merila M, Lutsar I. Comparison of ampicillin plus gentamicin vs. penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis. Acta Paediatr 2010;99:665-72.
- Leandro C, Leona WA. Duration of empiric antibiotics for suspected early onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol 2003;24(9):662-6.
- Labenne M, Michaut F, Gouyon B, Ferdynus C, Gouyon J-B. A population-based observational study of restrictive guidelines for antibiotic therapy in early onset neonatal infections. Pediatr Infect Dis J 2007;26(7):593-9.
- Gonzalez L, Cravoisy A, Barraud D, Conrad M, Nace L, Lemarie J, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care 2013;17:R140.
- Rao SC, Srinivasjois R, Hagan R, Ahmed M. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 2006;11:CD005091.
- Neonatal infection (Neonatal infection (early onset): antibiotics for pre-antibiotics for prevention and treatment Clinical Guideline. United Kingdom: National Institute for Health and Care Excellence (NICE); 2012. Available from: https://www.nice.org.uk/guidance/cg149.
- Ibrahim NA, Manan MM, Aziz NA. The effectiveness of empiric antibiotic therapy in the prevention of early onset sepsis. Int J Curr Res 2014;6(4):6258-65.
- Jeffrey S, Gerdes MD. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin N Am 2004;51:939-59.
- Chauhan N, Tiwari S, Jain U. Potential biomarkers for effective screening of neonatal sepsis infections: An overview. Microb Pathog 2017;107:234-42.
- Masterton RG. Antibiotic de-escalation. Crit Care Clin 2011;27:149-62.
- Garnacho-Mentero J, Escoresca-Ortega A, Fernandez-Delgado E. Antibiotic de-escalation in the ICU: how is it best done? Curr Opin Infect Dis 2015;28:193-8.
- Pasha YZ, Ahmadpour-Kacho M, Behmadi R, Jahangir T. 3-day versus 5-day course of intravenous antibiotics for suspected early onset neonatal sepsis: a randomized controlled trial. Iran J Pediatr 2014;24(6):673-78.
- Gelfand SL, Fanaroff JM, Walsh MC. Meconium stained fluid: approach to the mother and the baby. Pediatr Clin North Am 2004;51(3):655-67.
- Yurdakok M. Meconium aspiration syndrome: do we know? Turk J Pediatr 2011;53:121-9.
- Basu S, Kumar A, Bhatia BD. Role of antibiotics in meconium aspiration syndrome. Ann Trop Paediatr 2007;27:107-13.
- Muller-Pebody B, Johnson AP, Heath PT, Gilbert RE, Henderson KL, Sharland M. Empirical treatment of neonatal sepsis: are the current guidelines adequate? Arch Dis Child Fetal Neonatal Ed 2011;96:F4–F8.
- Cotten CM, Smith PB. Duration of empiric antibiotic therapy for infants suspected of early-onset sepsis. Curr Opin 2013;25(2):167-71.
- Oliver EA, Reagan PB, Slaughter JL, Buhimschi CS, Buhimschi IA. Patterns of empiric antibiotic administration for presumed early-onset neonatal sepsis in Neonatal Intensive Care Units in the United States. Am J Perinatol 2017;34(7):640-7.
- Ho JJ. Appropriate use of antibiotics in the NICU. East J Med 2010;26:133-8.
- Mukhopadhyay S, Eichenwald EC, Puopolo KM. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J Perinatol 2013;33:198-205.
- Ramesh BY, Lincy PB. Early onset of neonatal sepsis: Analysis of the risk factors and the bacterial isolates by using the BacT Alert System. J Clin Diag Res 2011;Suppl-2,5(7):1385-88.
- Zaidi AKM, Thaver D, Ali SA, Ahmed Khan T. Pathogens associated with sepsis in newborns and young infants. Pediatr Infect Dis J 2009;28:S10-S18.
 ) De-escalation I, (
) De-escalation I, ( ) de-escalation II
) de-escalation II



