- *Corresponding Author:
- Mei Yang
Department of Thoracic Surgery, West China Hospital of Sichuan University, Chengdu, Sichuan 610041, China
E-mail: yangmei69@126.com
| Date of Received | 09 June 2024 |
| Date of Revision | 27 July 2024 |
| Date of Acceptance | 06 August 2024 |
| Indian J Pharm Sci 2024;86(4):1355-1362 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Gastroesophageal reflux disease is a common digestive disorder often associated with medication use. This study aimed to identify top medications strongly associated with gastroesophageal reflux disease through the Food and Drug Administration's adverse event reporting system. Food and Drug Administration's adverse event reporting system database was queried for adverse event reports related to gastroesophageal reflux disease from 2013 to 2022. Disproportionality analysis was conducted using the reporting odds ratio and proportional reporting ratio. To identify instances of gastroesophageal reflux disease, the medical dictionary for regulatory activities was utilized and the DrugBank database was used to determine the generic nomenclature of the drugs. A total of 5470 cases of gastroesophageal reflux disease reports were identified. Overall, there is a higher likelihood of drug-induced gastroesophageal reflux disease in females. Benicar, Fosamax, Enbrel, Benicar HCT and Prolia were the top 5 drugs associated with most cases of gastroesophageal reflux disease. According to the disproportionality analysis, Benicar and its combination medications ranked as the top three drugs with the strongest gastroesophageal reflux disease signals. By analyzing the Food and Drug Administration's adverse event reporting system database, we have listed medications with a strong gastroesophageal reflux disease signal. Our research indicated that angiotensin II receptor blockers, particularly Benicar (olmesartan), might be a category of drugs with underestimated susceptibility to gastroesophageal reflux disease risk.
Keywords
Gastroesophageal reflux disease, Food and Drug Administration's adverse event reporting system database, disproportionate analysis, olmesartan
Gastroesophageal Reflux Disease (GERD) is a relatively common digestive disorder worldwide[1]. Due to a lack of effective diagnostic criteria for GERD, estimating its prevalence is challenging. When defined according to Montreal definition and classification, the prevalence of GERD in Western countries ranges between 10 % and 20 %[2,3]. Patients with GERD may experience heartburn, acid regurgitation and other persistent symptoms, significantly impacting their quality of life and work[4]. When patients exhibit extraesophageal manifestations, especially respiratory complications such as asthma-like attacks or even suffocation, it can pose a life-threatening risk[5]. Additionally, chronic GERD is associated with complications such as Barrett's esophagus and esophageal cancer [6].
Previous epidemiological studies indicate a potential link between GERD, smoking and coffee consumption, recognized as behavioral risk factors for triggering GERD symptoms[2]. Of note, the utilization of certain medications may contribute to the onset or worsening of GERD symptoms[7]. Prior research has identified various drug classes and specific medications linked to GERD, including bisphosphonates, acetylsalicylic acid, antidepressant agents, hormone replacement therapy and oral contraceptive drugs[8-11]. These medications may induce GERD through direct mucosal damage, inflammation or by reducing Lower Esophageal Sphincter Pressure (LESP) and influencing esophageal motility[12,13]. However, although the association between GERD and certain medications is frequently reported, limited clinical observations and retrospective studies are often insufficient to draw conclusions[14]. Furthermore, existing literature lacks large-scale studies on which class of drugs is most likely to induce GERD. Our study aims to address this issue through the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), a drug safety surveillance database.
FAERS, a self-reporting system, is created for the collection of post-marketing Adverse Events (AEs) associated with drugs and therapeutic biologics[15]. Owing to its attributes of vast data and public accessibility, FAERS is commonly employed for pharmacovigilance investigations. Previous research has employed the FAERS database to investigate correlations between specific drugs and various gastrointestinal AEs, such as bevacizumab and gastrointestinal perforations and glucagon-like peptide-1 receptor agonists and gastrointestinal adverse reactions[16,17]. However, the association between specific drugs and GERD remains elusive.
With the introduction of numerous new drugs in recent years, screening and updating their relevance to GERD are imperative. This study aims to comprehensively investigate drugs associated with an increased risk of GERD through FAERS. Such a list of medications could be instructive for prescribing physicians in managing iatrogenic GERD patients and provide valuable data for future explorations in more specific epidemiologic research.
Materials and Methods
Study design:
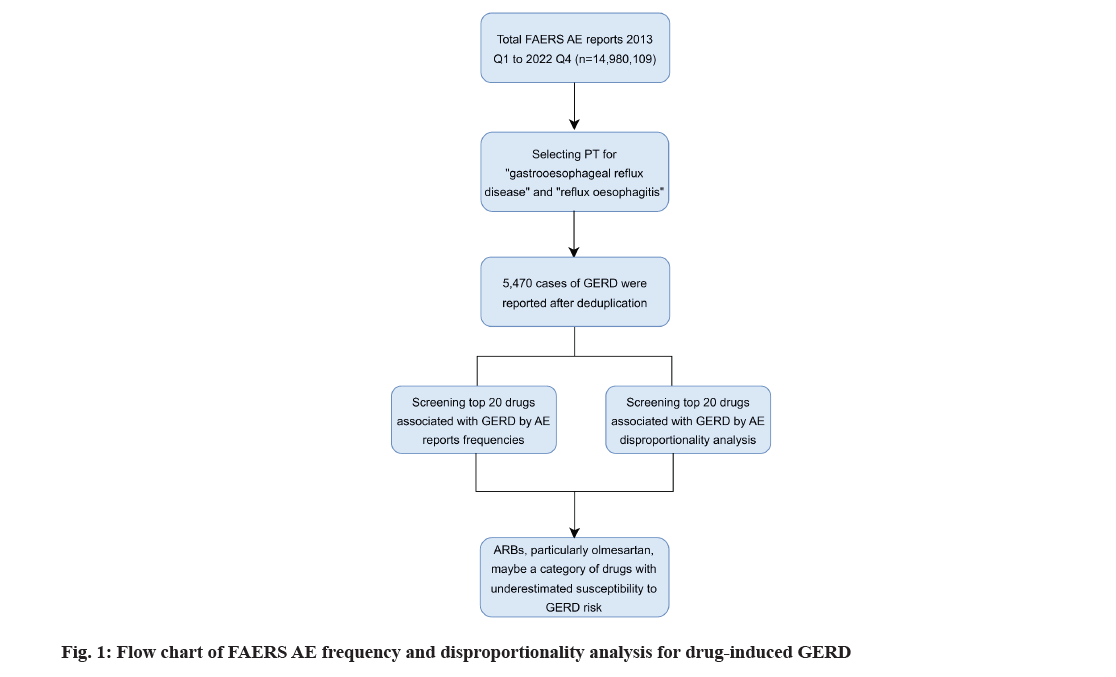
We divided this study into three stages; data collection and collation; data definition and translation; data analysis. Firstly, we extracted relevant data from the FAERS database for evaluation. Then, because both generic and brand drugs are recorded in FAERS, the subsequent translation stage converted all brand drugs in the project into their generic equivalents. Finally, during the data analysis stage, we obtained the 20 most common drugs reported in FAERS associated with GERD and their corresponding Reporting Odds Ratio (ROR).
Data source:
This pharmacovigilance research was conducted through retrospective analysis of the FAERS database. FAERS, designed to support post-marketing surveillance of drugs and therapeutic biologics, encompasses all adverse reaction and medication error information collected by the FDA[18]. All data in the database are voluntarily submitted by healthcare professionals and consumers, providing a wealth of information, including demographic characteristics, drug details and clinical outcome information. We utilized Open Vigil 2.1 pharmacovigilance analysis platform for data mining in the FAERS database (http:// h2876314.stratoserver.net:8080/OV2/search/). Open Vigil 2.1 currently includes FAERS data from Q1 2004 to Q1 2023, comprising a total of 11 155 106 AEs[19].
AEs and drugs definition:
Using the Preferred Term (PT) nomenclature from the Medical Dictionary for Regulatory Activities (MedDRA, version 24.0), we encoded the AE signals retrieved from the FAERS database. Then, we conducted data mining in the PT section for "GERD" and "reflux oesophagitis" (MedDRA codes: 10017885, 10017885) to identify drugs associated with GERD. We then downloaded all reports related to GERD and conducted statistical analyses using generic drug names as the unique identifiers. However, many reports in the FAERS database utilized brand names for drugs. To convert these brand names to generic names, we used the FDA DrugBank database (https://go.drugbank. com/drugs). If a drug name could not be retrieved from DrugBank, we manually excluded it from our analysis.
Statistical analysis:
Descriptive analysis was conducted to summarize the clinical characteristics of patients with drug-induced GERD, including age, gender, weight and relative outcomes. Individual safety reports (ISRs), with each ISR counting as one AE report, were tallied to identify the top 20 drugs associated with GERD (ranked in two groups based on the number of reported cases and ROR values). Disproportionate analysis was employed to generate hypotheses regarding potential associations between drugs and GERD[20]. Within disproportionate analysis, the ROR and Proportional Reporting Ratio (PRR) are the two most commonly used frequency methods, distinguished by their simplicity in calculation and consistency in results[21,22]. Table 1 outlines the calculation formulas and criteria. A relatively higher ROR or PRR indicates a stronger statistical relationship between the suspected drug and the suspected AE. Open Vigil 2.1 was utilized to directly compute ROR and PRR values, followed by data processing and analysis using the R programming language (version 4.3.1).
| Algorithms | Equation | Criteria |
|---|---|---|
| ROR | ROR = ad/bc | lower limit of 95 %CI >1, a≥3 |
| 95% CI=eln(ROR)±1.96(1/a+1/b+1/c+1/d)^0.5 | ||
| PRR | PRR=a(c+d)/c(a+b) | PRR≥2, χ2 ≥4, a≥3 |
| χ2=[(ad–bc)2](a+b+c+d)/[(a+b)(c+d)(a+c)(b+d)] |
Note: a: number of reports containing both the suspect drug and the suspect adverse event; b: number of reports containing the suspect adverse event with other medications (except the drug of interest); c: number of reports containing the suspect drug with other adverse drug reactions (except the event of interest); d: number of reports containing other drugs and other adverse events. ROR: Reporting Odds Ratio; PRR: Proportional Reporting Ratio; CI: Confidence Interval and χ2: chi-squared
Table 1: Algorithms used for signal detection
Results and Discussion
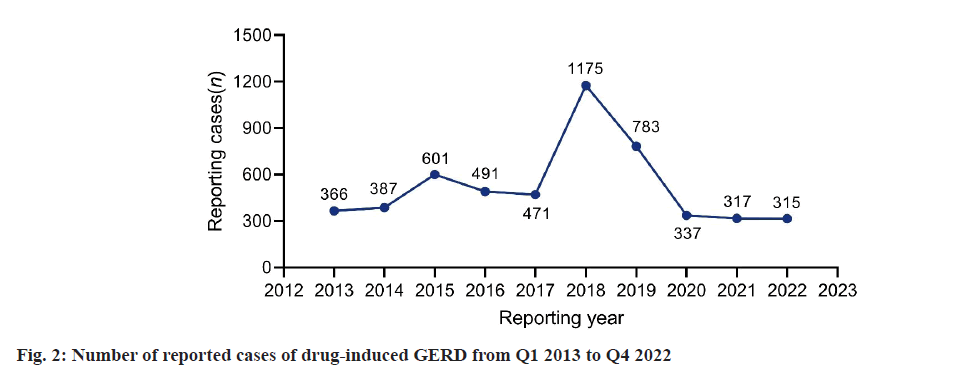
By limiting the reporters to physicians (abbreviated as MD) and the reporting countries to the United States, a total of 5470 cases of GERD were reported from the first quarter of 2013 to the fourth quarter of 2022. As shown in fig. 1, the number of reports of drug-induced GERD peaked at 1175 cases in 2018. Starting from 2019, the number of reports began to decline and the reported cases of GERD fell back to the level of 10 y ago from 2020 to 2022. According to the characteristics presented in Table 2, druginduced GERD is more likely to occur in individuals aged 41-64 (1076, 19.67 %) and ≥65 y old (870, 15.90 %), with lower incidences in individuals aged 19-40 (266, 4.86 %) and minors (118, 2.16 %). The average weight of patients with drug-induced GERD was 76 (±27.53) kg. Additionally, women are more susceptible to drug-induced GERD compared to men (n=2863, 52.34 % vs. n=1163, 21.26 %). Among these reports, the most common outcome was hospitalization-initial or prolonged (n=1616, 29.54 %).
| Characteristics | No. /Kg (%/SD) |
|---|---|
| Age | |
| ≤18 | 118 (2.16) |
| 19-40 | 266 (4.86) |
| 41-64 | 1076 (19.67) |
| ≥65 | 870 (15.90) |
| Unknown | 3140 (57.40) |
| Gender | |
| Male | 1163 (21.26) |
| Female | 2863 (52.34) |
| Unknown | 1444 (26.40) |
| Weight | 76 (27.53) |
| Outcome | |
| Hospitalization-Initial or prolonged | 1616 (29.54) |
| Life-Threatening | 65 (1.19) |
| Death | 135 (2.47) |
| Disability | 150 (2.74) |
| Required intervention to prevent | 3 (0.05) |
| Congenital anomaly | 70 (1.28) |
| Other Serious (Important Medical Event) | 2059 (37.64) |
| Unknown | 1372 (25.08) |
Note: Categoric data are shown as number (%) and continuous data as mean±standard deviation; GERD: Gastroesophageal Reflux Disease and Kg: Kilogram
Table 2: Clinical characteristics of reported drug-induced GERD.
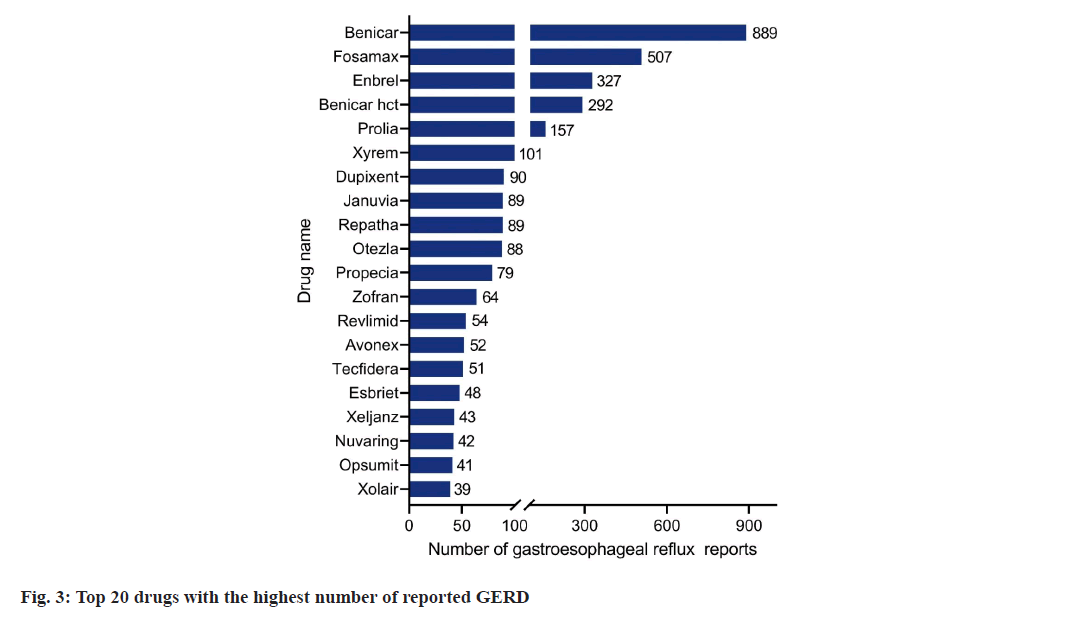
Based on the frequency of AE reports, the top 20 drugs associated with GERD are shown in fig. 2. Benicar is the drug with the highest reported frequency (889 cases), followed by Fosamax (507 cases), Enbrel (327 cases), Benicar HCT (292 cases), Prolia (157 cases), Xyrem (101 cases), Dupixent (90 cases) and other medications. In the frequency rank figure (fig. 2), drugs classified as biologics and immuno-modulators account for the largest proportion, followed by antihypertensive drugs and other drugs.
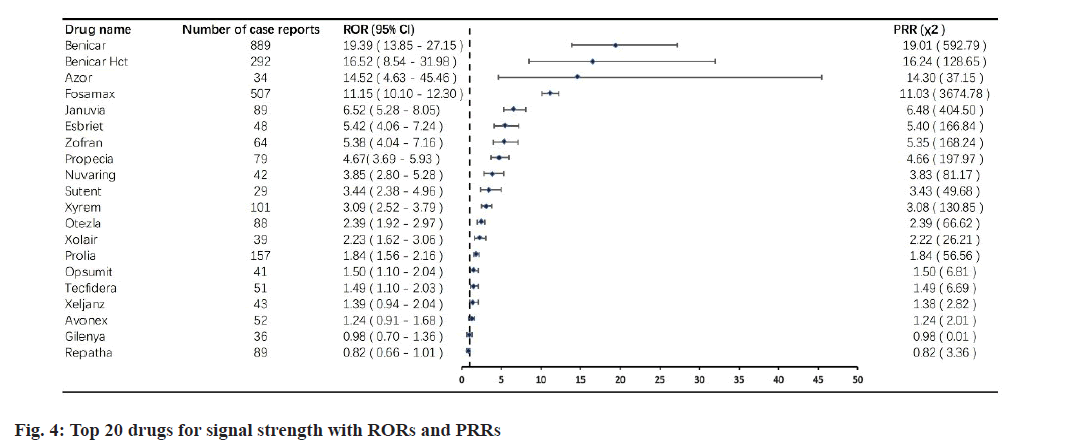
Based on the ROR criteria and reporting frequency, we have identified the top 20 drugs with GERD signal values. fig. 3 and fig. 4 lists the top 20 drugs with the highest signal strength, with ROR values ranging from 0.82 to 19.39. According to this list, Benicar remains the drug with the highest signal value (ROR 19.39, 95 % CI 13.85-27.15). The drugs that follow Benicar, including Benicar HCT (16.62, 95 % CI 8.54-31.98) and Azor (14.52, 95 % CI 4.63-45.46), are two-in-one drugs that contain Benicar. Fosamax, which had a high reporting frequency but ranked second, has a ROR value of 11.15, 95 % CI 10.10- 12.30. Enbrel, which also had a high ranking in terms of reporting frequency, did not make it into the top 20 drugs based on signal value. Similarly, using the POR criteria, we obtained a top 20 list of drugs that were consistent with the results obtained using the ROR criteria.
To our knowledge, this study is the first to generate a list of medications with the highest likelihood of causing GERD, based on analysis of real-world data from the FAERS database. We described the clinical characteristics of patients with drug-induced GERD and identified the drugs most associated with it. Our findings suggest that Benicar and its combination drugs may be under-recognized as potential inducers of GERD. This could be instructive for prescribing physicians in managing iatrogenic GERD patients and contribute to future clinical research.
GERD is a non-negligible AE to medical interventions. Prolonged and severe GERD can markedly diminish the quality of life. Previous studies have found that Angiotensin II Receptor Blockers (ARBs), bisphosphonates, hormone replacement therapy, immuno-modulators and selective serotonin reuptake inhibitors may be associated with drug-induced GERD, which is consistent with our findings[7,10,23,24]. Of note, our study demonstrates that the incidence of drug-induced GERD increases with age and is more common in female patients. Previous research has shown that hormonal changes associated with menopause may increase the susceptibility to mucosal injury and disturb gastrointestinal tissue repair[25]. Additionally, as people aging, their gastric mucosal resistance to injury decreases, which may increase the risk of drug-induced GERD.
The drugs most frequently associated with GERD among the top 20 ranked by the calculated ROR are predominantly immuno-modulators. Notably, a significant portion of these drugs is used in the treatment of autoimmune diseases, including Tecfidera, Xeljanz, Avonex and Gilenya. In previous reports, Xeljanz (Tofacitinib) has been mentioned in association with GERD or gastrointestinal perforation, particularly in STING-associated vasculopathy with onset in infancy and Rheumatoid Arthritis (RA) patients[26,27]. The remaining three drugs, Tecfidera, Avonex and Gilenya, commonly used in the treatment of multiple sclerosis have been previously reported to cause gastrointestinal adverse reactions in previous studies[28-30]. However, the specific association with GERD is not detaileddocumented. Our work may provide new insights for future research on the relationship between these anti-autoimmune disease drugs and GERD. Additionally, some postmenopausal females undergoing bisphosphonate therapy for osteoporosis have experienced severe esophageal injury[31]. Endoscopic examinations in these cases often reveal chemical esophagitis with erosion or ulcers, exudative inflammation and thickening of the esophageal wall. Furthermore, recent studies indicate that combined oral contraceptives are an independent risk factor for GERD[32]. Our research, however, demonstrates that NuvaRing is more likely to cause GERD compared to oral contraceptives. It is also worth noting that 5α-reductase inhibitors have previously been reported in studies as drugs that can reduce the risk of gastrooesophageal cancer. However, Propecia has also appeared in the list of top 20 drugs associated with GERD reports number and ROR values, the specific mechanism awaits further exploration. In the context of the relationship between 5-Hydroxytryptamine Type 3 (5-HT3) receptor inhibitors and GERD, the blockade of 5-HT3 receptors may lower midsternum somatic pain thresholds, leading to decreased pain thresholds after esophageal acid exposure, which may be associated with Zofran induced GERD[33]. The association between incretin-based drugs and GERD-like symptoms has recently been widely discussed[34]. Our study found that Januvia, a dipeptidyl peptidase-4 inhibitor, is more likely to induce GERD. This may have implications for the medication of patients with type 2 diabetes who already experience gastrointestinal discomfort.
ARBs stand as one of the most widely prescribed anti-hypertensive medications globally, which are routinely employed as first-line agents in hypertensive management for individuals with diabetes and renal disorders[35,36]. ARBs functioning by competitively inhibiting angiotensin II receptors, thereby lowering blood pressure and resulting in diminished vascular constriction, reduced aldosterone secretion and decreased release of catecholamines[37]. Notably, losartan was the pioneer ARB to receive approval, while olmesartan gained regulatory approval in 2002. In 2012, Rubio-Tapia et al.[38] reported a case series involving 22 hypertensive patients treated with olmesartan, who presented with unexplained chronic diarrhea and sprue-like enteropathy. Upon discontinuation of olmesartan, histological recovery was then observed, accompanied by symptoms relief. Following this case series, additional reports documented cases of sprue-like enteropathy associated with olmesartan medication[39,40]. Although the mechanism by which olmesartan induces mucosal damage remains unclear, it is commonly acknowledged that the process involves immunemediated inflammation. This immune-mediated damage manifests as partial to severe villous atrophy, increased intraepithelial lymphocytes, frequent subepithelial collagen deposition and inflammation in the lamina propria[41]. Our study is the first to reveal the potential association between olmesartan and its derivatives in the development of GERD. ARBs-related AEs of digestive tract should be considered a distinct clinical entity and included in the differential diagnosis of diarrhea in hypertensive patients. Moreover, further clinical and mechanistic investigations pertaining to the relationship between ARBs and GERD are warranted.
While this real-world pharmacovigilance study provides a reference for identifying drugs with potential risks inducing GERD, certain inevitable limitations should be noted. Firstly, the true incidence rates of GERD for each drug cannot be compared due to the unknown total number of cases for each medication. Secondly, given that the FAERS operates on a spontaneous reporting system, inherent challenges like underreporting, incomplete reporting and reporting bias are present, potentially introducing bias into the data analysis. Thirdly, the association signals identified in our study do not definitively establish a causal relationship between the drugs and GERD. Although our study does not delve into the specific impact of drugs on GERD, the FAERS database remains a crucial tool for pharmacovigilance analysis and provides clues for further prospective clinical investigations.
In summary, herein we conducted a comprehensive assessment of GERD reports and associated drugs in the FAERS database. Our research indicates that ARBs, particularly olmesartan, maybe a category of drugs with underestimated susceptibility to GERD risk, warranting close monitoring in medical practice. Furthermore, additional epidemiological investigations are needed to explore the associations between these drugs and GERD.
Author contributions:
Study concept and design; acquisition of data; interpretation of data; drafting and revision of the manuscript was done by Yu Chen. Study concept and design; analysis and interpretation of data; drafting and revision of the manuscript was done by Mei Yang. All authors approved the final version of the manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Mehta RS, Staller K, Chan AT. Review of gastroesophageal reflux disease. JAMA 2021;325(14):1472-.
- El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014;63(6):871-80.
[Crossref] [Google Scholar] [PubMed]
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101(8):1900-20.
[Crossref] [Google Scholar] [PubMed]
- Yadlapati R, Gyawali CP, Pandolfino JE, Chang K, Kahrilas PJ, Katz PO, et al. AGA clinical practice update on the personalized approach to the evaluation and management of GERD: Expert review. Clin Gastroenterol Hepatol 2022;20(5):984-94.
[Crossref] [Google Scholar] [PubMed]
- Pensabene L, Miele E, Del Giudice E, Strisciuglio C, Staiano A. Mechanisms of gastroesophageal reflux in children with sequelae of birth asphyxia. Brain Dev 2008;30(9):563-71.
[Crossref] [Google Scholar] [PubMed]
- Nicholson A, Jankowski J. Acid reflux and oesophageal cancer. In: Inflammation and Gastrointestinal Cancers. Recent Results Cancer Res 2011;185:65-82.
[Crossref] [Google Scholar] [PubMed]
- Mungan Z, P?narba?? ?im?ek B. Which drugs are risk factors for the development of gastroesophageal reflux disease? Turk J Gastroenterol 2017;28(Suppl 1):S38-s43.
[Crossref] [Google Scholar] [PubMed]
- Martin-de-Argila C, Martinez-Jimenez P. Epidemiological study on the incidence of gastroesophageal reflux disease symptoms in patients in acute treatment with NSAIDs. Expert Rev Gastroenterol Hepatol 2013;7(1):27-33.
[Crossref] [Google Scholar] [PubMed]
- Jacobson BC, Moy B, Colditz GA, Fuchs CS. Postmenopausal hormone use and symptoms of gastroesophageal reflux. Arch Intern Med 2008;168(16):1798-804.
[Crossref] [Google Scholar] [PubMed]
- Ettinger B, Pressman A, Schein J. Clinic visits and hospital admissions for care of acid-related upper gastrointestinal disorders in women. Am J Manag Care 1998;4:1377-82.
[Google Scholar] [PubMed]
- Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: A double?blind, placebo?controlled study. Aliment Pharmacol Ther 2006;23(3):365-70.
[Crossref] [Google Scholar] [PubMed]
- Singh S, Bailey RT, Stein HJ, DeMeester TR, Richter JE. Effect of alprazolam (Xanax) on esophageal motility and acid reflux. Am J Gastroenterol 1992;87(4):483-488.
[Google Scholar] [PubMed]
- Lacy BE, Mathis C, DesBiens J, Liu MC. The effects of nebulized albuterol on esophageal function in asthmatic patients. Dig Dis Sci 2008;53:2627-33.
[Crossref] [Google Scholar] [PubMed]
- Brahm N, Kelly-Rehm M. Antidepressant-mediated gastroesophageal reflux disease. Consult Pharm 2011;26(4):274-8.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Zhou P, He N, Zhai S. Drug-induced torsades de pointes: Disproportionality analysis of the United States Food and Drug Administration adverse event reporting system. Front Cardiovasc Med 2022;9:966331.
[Crossref] [Google Scholar] [PubMed]
- Wichelmann TA, Abdulmujeeb S, Ehrenpreis ED. Bevacizumab and gastrointestinal perforations: A review from the FDA Adverse Event Reporting System (FAERS) database. Aliment Pharmacol Ther 2021;54(10):1290-7.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol 2022;13:1043789.
[Crossref] [Google Scholar] [PubMed]
- Duggirala HJ, Tonning JM, Smith E, Bright RA, Baker JD, Ball R, et al. Use of data mining at the Food and Drug Administration. J Am Med Inform Assoc 2016;23(2):428-34.
[Crossref] [Google Scholar] [PubMed]
- Böhm R, Höcker J, Cascorbi I, Herdegen T. OpenVigil—free eyeballs on AERS pharmacovigilance data. Nat Biotechnol 2012;30(2):137-8.
[Crossref] [Google Scholar] [PubMed]
- Caster O, Aoki Y, Gattepaille LM, Grundmark B. Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: Recommendations for limiting false-positive associations. Drug Saf 2020;43:479-87.
- Ooba N, Kubota K. Selected control events and reporting odds ratio in signal detection methodology. Pharmacoepidemiol Drug Saf 2010;19(11):1159-65.
[Crossref] [Google Scholar] [PubMed]
- Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001;10(6):483-6.
[Crossref] [Google Scholar] [PubMed]
- Van Soest EM, Dieleman JP, Siersema PD, Schoof L, Sturkenboom MC, Kuipers EJ. Tricyclic antidepressants and the risk of reflux esophagitis. Am J Gastroenterol 2007;102(9):1870-7.
[Crossref] [Google Scholar] [PubMed]
- Taha AS, McCloskey C, Prasad R, Bezlyak V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): A phase III, randomised, double-blind, placebo-controlled trial. Lancet 2009;374(9684):119-25.
[Crossref] [Google Scholar] [PubMed]
- Grishina I, Fenton A, Sankaran-Walters S. Gender differences, aging and hormonal status in mucosal injury and repair. Aging Dis 2014;5(2):160-9.
[Crossref] [Google Scholar] [PubMed]
- Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: Risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol 2016;68(11):2612-7.
[Crossref] [Google Scholar] [PubMed]
- Tang X, Xu H, Zhou C, Peng Y, Liu H, Liu J, et al. STING-associated vasculopathy with onset in infancy in three children with new clinical aspect and unsatisfactory therapeutic responses to tofacitinib. J Clin Immunol 2020;40:114-22.
[Crossref] [Google Scholar] [PubMed]
- Vermersch P, Waucquier N, Michelin E, Bourteel H, Stojkovic T, Ferriby D, et al. Combination of IFNβ?1a (Avonex®) and mycophenolate mofetil (Cellcept®) in multiple sclerosis. Eur J Neurol 2007;14(1):85-9.
[Crossref] [Google Scholar] [PubMed]
- Naismith RT, Wundes A, Ziemssen T, Jasinska E, Freedman MS, Lembo AJ, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing–remitting multiple sclerosis: Results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs 2020;34:185-96.
[Crossref] [Google Scholar] [PubMed]
- Guarnera C, Bramanti P, Mazzon E. Comparison of efficacy and safety of oral agents for the treatment of relapsing–remitting multiple sclerosis. Drug Des Dev Ther 2017:2193-207.
[Crossref] [Google Scholar] [PubMed]
- Strampel W, Emkey R, Civitelli R. Safety considerations with bisphosphonates for the treatment of osteoporosis. Drug Saf 2007;30:755-63.
[Crossref] [Google Scholar] [PubMed]
- Saleh S, Liu BD, Trujillo S, Thomas C, Fass R. The effect of combined oral contraceptives and Nexplanon on gastroesophageal reflux disease in premenopausal women: A nationwide database analysis. Neurogastroenterol Motil 2023;35(5):e14542.
[Crossref] [Google Scholar] [PubMed]
- Szczesniak MM, Fuentealba SE, Zhang T, Cook IJ. Modulation of esophageal afferent pathways by 5?HT3 receptor inhibition. Neurogastroenterol Motil 2013;25(5):383-e293.
[Crossref] [Google Scholar] [PubMed]
- Noguchi Y, Katsuno H, Ueno A, Otsubo M, Yoshida A, Kanematsu Y, et al. Signals of gastroesophageal reflux disease caused by incretin-based drugs: A disproportionality analysis using the Japanese adverse drug event report database. J Pharm Health Care Sci 2018;4:1-8.
[Crossref] [Google Scholar] [PubMed]
- Zhao D, Liu H, Dong P. A meta-analysis of antihypertensive effect of telmisartan versus candesartan in patients with essential hypertension. Clin Exp Hypertens 2019;41(1):75-9.
[Crossref] [Google Scholar] [PubMed]
- Fuchs FD, DiNicolantonio JJ. Angiotensin receptor blockers for prevention of cardiovascular disease: Where does the evidence stand? Open Heart 2015;2(1):e000236.
[Crossref] [Google Scholar] [PubMed]
- Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. In: Baylor University Medical Center Proceedings 2003;16(1):123-6).
[Crossref] [Google Scholar] [PubMed]
- Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wu TT, et al. Severe spruelike enteropathy associated with olmesartan. In: Mayo clinic proceedings 2012;87(8):732-8.
[Crossref] [Google Scholar] [PubMed]
- Ianiro G, Bibbò S, Montalto M, Ricci R, Gasbarrini A, Cammarota G. Systematic review: Sprue?like enteropathy associated with olmesartan. Aliment Pharmacol Ther 2014;40(1):16-23.
[Crossref] [Google Scholar] [PubMed]
- Briongos-Figuero LS, Cuevas-González J. Olmesartan-associated sprue-like enteropathy. Lancet 2023;402(10413):1660.
[Crossref] [Google Scholar] [PubMed]
- Marietta EV, Nadeau AM, Cartee AK, Singh I, Rishi A, Choung RS, et al. Immunopathogenesis of olmesartan?associated enteropathy. Aliment Pharmacol Ther 2015;42(11-12):1303-14.
[Crossref] [Google Scholar] [PubMed]