- *Corresponding Author:
- Zhenyan Bo
Department of Pharmacy, West China Second University Hospital, Sichuan University, Children's Medicine Key Laboratory of Sichuan Province, Sichuan 610000, China
E-mail: bozhenyanyh@stu.scu.edu.cn
| Date of Received | 27 August 2024 |
| Date of Revision | 02 September 2024 |
| Date of Acceptance | 09 September 2024 |
| Indian J Pharm Sci 2024;86(5):1725-1733 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Drug shortages have posed clinical and economic challenges to healthcare systems globally. Multiple data-based evidence on drug shortages in China is limited. This study aimed to depict drug shortage and cause of shortage in Southwest China and it was a cross-sectional study. Data was extracted from the multi-source drug shortage reporting system of Sichuan Province from 1st January, 2018 to 30th May 2021. The policy characteristics, dosage forms, shortage frequency, anatomical therapeutic chemical classification and cause for the drug shortage was analysed. There were 2184 reported drug shortages involving 870 drugs. Among the drugs, 631 (72.53 %) were chemical drugs. Injections had the highest average shortage frequency of 4.35, while 59.54 % (518/870) of the drugs in shortage was oral. All anatomical therapeutic chemical classification of drugs had been reported in shortage. The number of drugs for alimentary tract and metabolism was up to 117. As for policy characteristics, 44.48 % (387/870) and 79.54 % (692/870) fell into the national essential medicines list and national medical insurance drug list respectively. The incidence of drug shortage on the emergency drug list and national essential medicines list was significantly higher than that of the paediatric generic drug list and national medical insurance drug list (p<0.05). Manufacturing problems was the main causes of drug shortage (46.10 %, 891/1998). A plethora of drugs experienced a shortage in Southwest China, encompassing nearly the entirety of the anatomical therapeutic chemical classification system. Drug shortage affected the entire drug supply chain, including production, delivery and utilization. To address this issue, it was imperative to foster enhanced collaboration among all stakeholders to mitigate and resolve the drug shortage problem.
Keywords
Dosage form, epidemiology, ketamine, cardiovascular system, gynaecology, urokinase
Drug shortages have become a growing global concern, posing economic and clinical challenges to healthcare systems in high, middle and lowincome countries[1-3]. For patients, drug shortage is an impediment to the availability of appropriate therapeutic drugs, leading to increasing health care expenditure and unsatisfactory treatment outcome[4-6]. In certain extreme cases, drug shortages can even pose a threat to patient’s life[7,8]. For medical personnel, drug shortages may require extra time and increase medication errors due to changes in treatment strategy[9-11]. In a survey conducted by European Association of Hospital Pharmacists (EAHP) from 2019 to 2020, 95 % of hospital pharmacists, 71 % of physicians, 62 % of nurses and 89 % of other healthcare professionals considered medicine shortages as a significant obstacle to providing optimal medical care to patients[12]. For both the healthcare system and society, the occurrence of drug shortages would lead to an overall increase in healthcare expenditure[13,14].
China has made considerable progress in the development of its drug supply system over the past few decades. In 2017, the National Health and Family Planning Commission of the People's Republic of China (now renamed as the National Health Commission of the People's Republic of China) and other eight ministries jointly issued the implementation opinions on reforming and improving the supply security mechanism of drugs in shortage, which clearly taken hierarchical coping, classified management, consultation and linkage mechanism, secured supply as the principle for solving the drug shortage problem[15]. In 2019, the General Office of the State Council of the People’s Republic of China issued the further work to improve the supply and price stabilization of drugs in shortage. In this official government document, relevant policies about drug procurement, rational use, stockpiling system and price regulation were further optimized[16]. Till date, China has not experienced widespread or long-lasting drug shortages. The variety and quantity of drugs in China have generally been sufficient to meet clinical demand. Nevertheless, in recent years, localized or temporary drug shortages have continued to occur[17].
A quantitative description of the frequency, duration, intensity and other characteristics of drug shortage can not only provide a comprehensive understanding of the current situation of drug shortages, but also be the first and critical step to deal with the problem[13]. In order to achieve the aforementioned objectives, it was deemed that national or regional drug shortage surveillance data represented the optimal source for conducting such studies. However, such data-based studies on the current situation of drug shortages were very limited in China. Therefore, the objective of this study was to assess the characteristics of drug shortages based on data from the multi-source drug shortage reporting system in Sichuan Province from January 2018 to May 2021. We analysed the shortage frequency, dosage forms, Anatomical Therapeutic Chemical (ATC) classification, policy characteristics and causes of shortage to provide a descriptive overview of drug shortage in Southwest China. Furthermore, the study explored potential mitigation strategies and solutions to drug shortages.
Materials and Methods
Research site and data source:
This was a cross-sectional study and it was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist[18]. This study took Sichuan Province as research site, which is located in the Southwest of China. We extracted the data from the multi-source drug shortage reporting system of Sichuan province from 1st January, 2018 to 30th May, 2021. The system consisted of four data reporting sources including; the sentinel hospital monitoring network for drug shortages in Sichuan Province, which included 126 hospitals covering tertiary hospitals, secondary hospitals and primary medical institutions. The members of the Sichuan drug shortage consultation linkage mechanism, including the Health Commission of Sichuan Provincial, the Sichuan Provincial Economic and Information Department, the Sichuan Provincial Department of Commerce, The Administration for Market Regulation of Sichuan Provincial, The Sichuan Provincial Healthcare Security Administration, The Sichuan Provincial Administration of Traditional Chinese Medicine, The Sichuan Medical Products Administration and the Municipal (State) Health Commission of Sichuan Province and Pharmaceutical Manufacturers.
Generic name, dosage form, cause of shortage, and reporting source were directly extracted from each reported drug shortage. In addition, another six characteristics were considered of the reported drugs in shortage; the ATC classification maintained by World Health Organisation (WHO). The 2018 edition of the National Essential Medicines List (NEML)[19]. The 2021 edition of the National Medical Insurance Drug List (NMIDL)[20]. The list of demonstration drugs of directly online purchased emergency (rescue) drugs (chemicals and biological products) released in 2015 (hereinafter referred as the Emergency Drug List (EDL))[21]. The list of demonstration drugs of directly online purchased paediatric generic drugs (chemicals and biological products) released in 2015 (hereinafter referred as the Paediatric Drug List (PDL))[21]. The list of demonstration drugs of directly online purchased obstetrical and gynaecological generic drugs (chemicals and biological products) released in 2015 (hereinafter referred as the Obstetrical and Gynaecological Drug List (OGDL))[21]. In the meantime, drugs were classified as chemical drugs, Chinese traditional medicines, biological products, health care products according to the initial letter of drug approval number issued by the national medical products administration[22]. To ensure data quality, the generic names were verified individually through searching the official online drug inquiry platform of the national medical products administration[22].
Statistical analysis:
The number of reported shortage and drugs was counted for different drug type, dosage form and ATC classification. The Average Shortage Frequency (ASF) was then calculated using the following formula:
ASF=The number of reported shortages/the number of drugs
Comparing all the reported drugs in shortage with the NEML, NMIDL, EDL, PDL and OGDL, the proportions of drugs in shortage in each list was calculated. The Chi-square test was used to test whether there was difference in the proportions. The p≤0.05 was considered significant and was adjusted by Bonferroni when comparing pairwise. Data input and cleaning was completed using Microsoft Excel 2016. IBM Statistical Package for the Social Sciences (SPSS) for Windows 20 was used for statistical analysis.
Results and Discussion
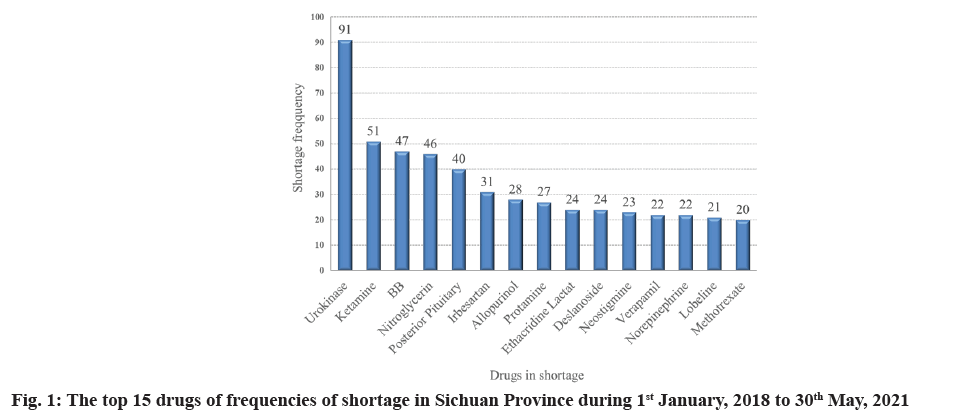
From 1st January, 2018 to 30th May, 2021, a total of 2184 drug shortages were reported. Among the multiple data source, tertiary sentinel hospitals reported the most drug shortage (651, 29.69 %) (Table 1). In total, 870 drugs were identified as being affected by the reported shortages. The ASF was 2.51 (2184/870). In terms of each drug, the shortage frequency for urokinase was upto 91, followed by ketamine (52), benzathine benzylpenicillin (47), nitroglycerin (46) and posterior pituitary (40) (fig. 1).
| Data source | Reported drug shortages (n=2184) |
|---|---|
| The sentinel hospital of drug shortage monitoring | |
| Tertiary hospitals | 644 (29.49) |
| Secondary hospitals | 301 (13.78) |
| Primary medical institutions | 319 (14.61) |
| Members of Sichuan drug shortage linkage mechanism | |
| The Sichuan provincial healthcare security administration | 421 19.28) |
| The Sichuan medical products administration | 61 (2.79) |
| The Sichuan provincial department of commerce | 31 (1.42) |
| The municipal (state) health commission | 314 (14.38) |
| Pharmaceutical manufacturers | 93 (4.26) |
Table 1: Data Sources of Drug Shortage in Sichuan Province from 1st January 2018 to 30th May 2021 (N, %)
Among all the drugs, chemical drugs and Chinese traditional medicines accounted for 97.24 % in total, with 72.52 % (631/870) for chemical drugs and 24.71 (215/870) for Chinese traditional medicines. Biological products and health care products only accounted for 2.41 % and 0.34 % respectively. The ASF of chemical drugs was 2.95, which was much higher than other drug types. As for dosage forms, 59.54 % (518/870) of the drugs in shortage was oral dosage, followed by injectable (27.82 %, 242/870), other (6.21 %, 56/870) and external dosage (6.21 %, 54/870). Notably, the ASF of injectable dosage was upto 4.35, higher than any other dosage forms (Table 2).
| Characteristics | Reported drug shortages (n, %) (n=2184) | Number of drugs (n, %) (n=870) | ASF |
|---|---|---|---|
| Type | |||
| Chemical drugs | 1861 (85.21) | 631 (72.53) | 2.95 |
| Chinese traditional medicines | 279 (12.77) | 215 (24.71) | 1.3 |
| Biological products | 40 (1.83) | 21 (2.41) | 1.9 |
| Health care products | 4 (0.18) | 3 (0.34) | 1.33 |
| Dosage form | |||
| Injectable | 1053 (48.21) | 242 (27.82) | 4.35 |
| Oral | 959 (43.91) | 518 (59.54) | 1.85 |
| External | 86 (3.94) | 54 (6.21) | 1.59 |
| Other | 86 (3.94) | 56 (6.44) | 1.54 |
Table 2: Type and Dosage Form of Drugs in Shortage in Sichuan Province from 1st January 2018 to 30th May 2021
In accordance with the ATC classification maintained by WHO, 631 chemical drugs and 21 biological products in shortage were classified into all the 14 categories. The number of drugs for alimentary tract and metabolism was upto 117, accounting for 17.94 % (117/652). The ASF for each drug belonging to cardiovascular system, blood and blood forming organs, systemic hormonal preparations, excl. Sex hormones and insulin was >4. The top 3 drugs in shortage in each category were shown in Table 3.
| ATC code | Reported drug shortages (n, %) | Number of drugs (n, %) | ASF | Top 3 drugs in shortage |
|---|---|---|---|---|
| C | 338 (17.78) | 68 (10.43) | 4.97 | Nitroglycerin, irbesartan and deslanoside |
| B | 281 (14.78) | 60 (9.20) | 4.68 | Urokinase, ethacridine lactate and arginine hydrochloride |
| N | 227 (11.94) | 67 (10.28) | 3.39 | Ketamine, neostigmine and diazepam |
| J | 219 (11.52) | 94 (14.42) | 2.33 | Benzathine benzylpenicillin, oseltamivir and protionamide |
| A | 205 (10.78) | 117 (17.94) | 1.75 | Vitamin AD and famotidine calcium gluconate |
| R | 139 (7.31) | 54 (8.28) | 2.57 | Lobeline, nikethamide and budesonide |
| L | 123 (6.47) | 39 (5.98) | 3.15 | Methotrexate, cytarabine and celecoxib |
| V | 82 (4.31) | 23 (3.53) | 3.57 | Protamine, pralidoxime and naloxone |
| H | 81 (4.26) | 18 (2.76) | 4.5 | Posterior pituitary, oxytocin and thiamazole |
| G | 66 (3.47) | 37 (5.67) | 1.78 | Conjugated estrogens, chlorquinaldol and estriol |
| M | 59 (3.10) | 23 (3.53) | 2.57 | Allopurinol, ibuprofen and colchicine |
| S | 36 (1.89) | 26 (3.99) | 1.38 | Tobramycin dexamethasone, sodium cromoglicate and diclofenac sodium |
| D | 35 (1.84) | 23 (3.53) | 1.52 | Calamine, ketoconazole and sulphur ointment |
| P | 10 (0.53) | 3 (0.46) | 3.33 | Albendazole, lindane and pyrimethamine |
Note: The total number of reported drug shortages was 1901 and the total number of drugs was 652, (C): Cardiovascular system; (B): Blood and blood forming organs; (N): Nervous system; (J): Anti-infective for systematic use; (A): Alimentary tract and metabolism; (R): Respiratory system; (L): Antineoplastic and immunomodulating agents; (V): Various; (H): Systemic hormonal preparations, excl. sex hormones and insulin; (G): Genito urinary system and sex hormones; (M): Musculo-skeletal system; (S): Sensory organs; (D): Dermatologicals and (P): Antiparasitic products, insecticides and repellents
Table 3: ATC Classification of Drugs in Shortage in Sichuan Province from 1st January 2018 to 30th May 2021
Among the 870 drugs, (44.48 %) and 79.54 % (692/870) of the drugs fell into the NEML and NMIDL respectively. The incidence of drug shortage on NEML was significantly higher than that of NMIDL (p<0.05). In addition, 72.88 % (43/59) of the drugs on EDL experienced shortage, which was significantly higher than that of PDL (33.93 %, p<0.05) (Table 4).
| Lists | Number of drugs (n, %) (n=870) | Ratio (%) |
|---|---|---|
| NEML | ||
| Essential medicine | 387 (44.48) | 387/685 (56.50 %)b |
| Non-essential medicine | 483 (55.52) | - |
| NMIDL | ||
| Medical insurance drug | 692 (79.54) | 692/2860 (24.20 %)a |
| Non-medical insurance drug | 178 (20.46) | - |
| EDL | ||
| Emergency drug | 43 (4.94) | 43/59 (72.88 %)b |
| Non-emergency drug | 827 (95.06) | - |
| PDL | ||
| Pediatric generic drug | 19 (2.18) | 19/56 (33.93 %)a |
| Non-pediatric generic drug | 851 (97.82) | - |
| OGDL | ||
| Obstetrical and gynecological generic drug | 9 (1.03) | 9/22 (40.10 %)a,b |
| Non-obstetrical and gynecological generic drug | 861 (98.97) | - |
Note: a,bp<0.05
Table 4: Drug Shortages in Sichuan Province on Each List from 1st January 2018 to 30th May 2021
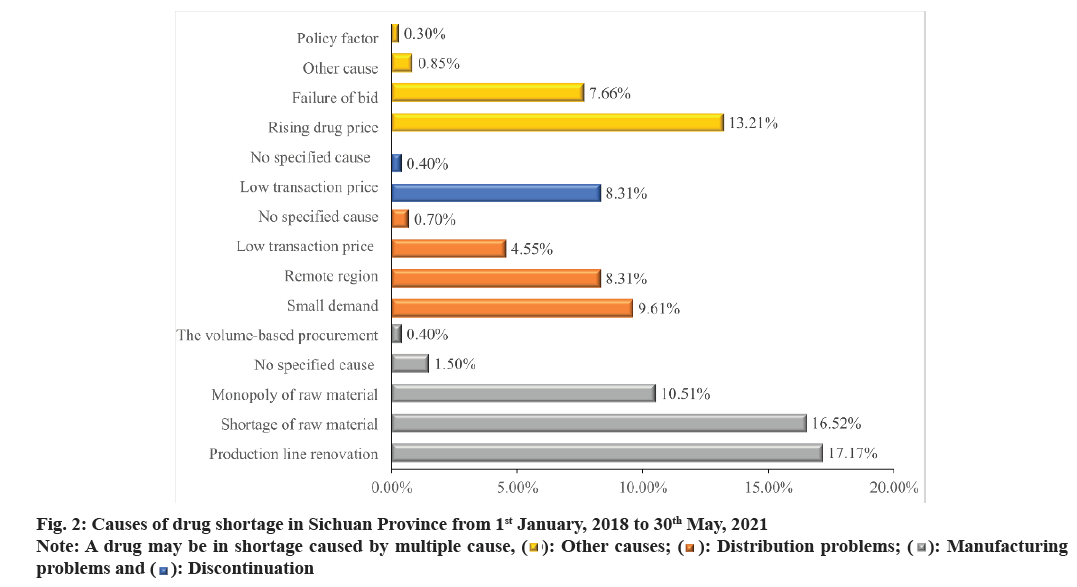
Among the reported drug shortages, 441 did not report the cause of shortage, and 242 reported multiple causes. Ultimately, 1998 causes of shortage were identified. The causes of shortage could be identified into 4 principal categories; manufacturing problems, distribution problems, discontinuation and other. The most reported cause was manufacturing problems (46.10 %, 891/1998), and the specific reason for insufficient production capacity included production line renovation (17.17 %, 343/1998), shortage (16.52 %, 330/1998) and monopoly (10.51 %, 210/1998) of raw material, and large demand led by the volume-based procurement (0.40 %, 8/1998). The 2nd most common reported cause was distribution problem (23.17 %, 463/1998), including pharmaceutical supplier’s reluctance to supply drugs because of small drug demand (9.61 %, 192/1998), remote region (8.31 %, 166/1998) and low transaction price (4.55 %, 91/1998). Discontinuation accounted 8.71 % (174/1998) of the reported cause of shortage. In addition, rising drug price and failure of bid played an important role in drug shortages, with 13.21 % (264/1998) and 7.66 % (153/1998) respectively (fig. 2).
There were 2184 shortages reported by different data source from January 2018 to 30th May, 2021 in Sichuan Province, which included 870 drugs with the ASF of 2.51 per drug. Compared with other provinces, the ASF of Sichuan Province was low. Ma et al.[23] found the ASF of Hubei province was 3.81 (2439/640), and in another literature, the ASF of Liaoning was up to 3.83 (964/252)[24].
In this study, drug shortages occurred at all levels of hospitals in Sichuan Province. Especially, it was more serious in tertiary hospitals than in other levels of hospitals. This may be caused by the great demand for drugs in tertiary hospitals[25]. Patients in China always tend to go to higher level of medical institutions regardless of the severity of disease[26]. From the whole drug supply chain, manufacturers should be the first to know an impending drug shortage, then the supplier, and finally the demand-side like hospitals. In other words, manufacturers should be more active and take more responsibility for reporting drug shortages to reserve sufficient time to develop contingency plans for related stakeholders. However, in our study, reported shortages from sentinel hospitals was far more than other reporting sources, just 4.26 % (93/2184) from pharmaceutical manufacturers. This definitely leads to poor timeliness of drug shortage information and make it more difficult to restore the supply after the market disruptions[27]. In China, some measures have been developed to improve the timeliness of drug shortage, including the measures for supervision and administration of drug production issued in 2020 which requires the holder of a pharmaceutical manufacturing license to report the unplanned drug halted production in 3 d[28], and the multi-source information collection platform for drug shortages launched in November 2021 to collect information of drug shortage from different stakeholders. But according to this study, the measures described above might not had the desired effect. Therefore, we proposed the establishment of a stricter monitoring mechanism for drug shortages and appropriate disclosure of information on drug shortages.
According to previous literature, the dosage form most likely to be in short supply seemed to be inconsistent so far. In the United States of America (USA), parenteral (61.2 %) was the most drugs in shortage from 2014 to 2019[29]. In France, injectable took upto 47.5 % of the total drugs in shortage from 2012 to 2018, which was higher than oral (43.3 %)[30]. But in contrast, the proportion of oral (51 %) was higher than injectable (40 %) in Europe[31]. In terms of the total number of drugs, our study was consistent with Europe, with the largest proportion of drugs in shortage being oral medicines. The underlying cause may be the great demand in clinical use of oral. But in terms of ASF, injectable was much higher than that of other dosage forms in this study, indicating higher shortage frequency of injectable. This may be partly explained by the strict Good Manufacturing Practice (GMP) for injectable. The manufacturing process of injectable was more complex than other dosage forms, so it is generally acknowledged that injectable was more susceptible to quality defects and production problem[31-33]. Data from the Food and Drug Administration (FDA) showed that quality-related or manufacture-related shortages of injectable had been upto 75 %[34].
As for therapeutic properties, nearly all ATC classification of drugs had been reported in shortage, but the severity of drug shortage in different ATC classifications was inconsistent. Studies in other countries or regions have showed similar results, although the specific ATC classification rank may not be the same[1,29,35-37]. According to this study, the cardiovascular system was the most serious classification in drug shortage with the ASF of 4.97. Nitroglycerin, as one of the most commonly used firstline drugs for acute ischemic heart disease, once it is in shortage, thousands of patients may experience serious health consequences[38]. According to the University of Utah Drug Information Service (UUDIS) database, the shortages of cardiovascular drugs always remained a fairly constant high level[39]. In consideration of the increasing prevalence of cardiovascular disease, ongoing shortages of cardiovascular drugs may become a public health problem[40,41]. Although the ASF of alimentary tract and metabolism drugs was low, the number of drugs experiencing shortage was the most. The underlying reason may be the dietary uptake of high salt in Sichuan Province[42].
In this study, 44.48 % (387/870) of the drugs in shortage were essential medicines, accounting for upto 56.50 % of the NEML, indicating that the shortage of essential medicines in Sichuan Province was quite severe. This was consistent with previous studies in China. A qualitative study conducted in Shaanxi Province showed that most drug shortage reported in the survey was essential medicines (51/95)[43]. Yinyin found that 51.0 % (208/408) of the drugs on the provincial drug shortage lists were essential medicines[44]. Such a high proportion of essential medicines in shortage greatly decreased the accessibility of these drugs for the public. In addition, 779.54 % (692/870) of the reported drugs in shortage were medical insurance drugs. Under such conditions, the patients and physicians had to seek for alternative drugs, which were generally much more expensive, increasing the economic burden of disease. Therefore, more attention should be paid to the adequate supply of these drugs.
Like other studies, this study also found a high ratio of emergency drugs in shortage. An online survey included 236 emergency physicians in China showed that drug shortages occurred every month or more frequently reported by 65.7 % of the respondents[25]. In the USA, nearly all the 30 commonly used emergency drugs experienced a shortage from 2006 to 2019[45]. If emergency medications were unavailable, the quality of medical care would not be guaranteed, patients could experience medication errors, treatment delays, adverse outcomes, and increased healthcare costs[46]. Drug accessibility for vulnerable groups have been of great concern. According to the drug shortage data from January 2001 to December 2015 obtained from UUDIS, 41.37 % (779/1883) of the products on shortage were used in paediatric emergency or critical care[47]. Raw found that 62.20 % (209/336) of medications on the 2019 WHO model list of essential medicines for children had been in shortage from 2014 to 2019[29]. Based on previous studies, the shortage of paediatric drugs in China was quite severe[48], which was also confirmed in this study with the drug shortage incidence of PDL of 33.93 %. In addition, the drug shortage incidence of OGDL was 40.10 % (9/22) in this study, indicating that pregnant women were another vulnerable group to experience drug shortage. Nixon, hold that generic drugs used in obstetric anaesthesiology were more susceptible to drug shortage[49]. From the bioethical and moral perspective, children and pregnant women have a special claim to social protection and consideration, more management strategies should be well established to ensure the availability of paediatric and obstetric drugs and related products.
The causes of drug shortage were multifaceted and complex, and it could only be analysed summarily in this study. As in other studies, manufacturing problems topped the list[32]. Raw material problems have been widely considered as one of the main reasons leading to manufacturing problem[50]. In consistent with previous studies[27,51], this study also found that volume-based drug procurement might lead to emergence of drug shortages, the underlying reason may be a sudden increase in demand for disease-specific drugs. For other three groups of cause, the root cause behind was about economic benefit[31,52-54]. At present, the most common and important way to get access to China's drug supply chain was to win the competitive public biding.
Meanwhile, the bid-wining prices were not allowed to be higher than the ceiling price set by government sectors, so the marketing of the drug became less profitable and less attractive for manufacturers to win the bid, which may lead to decreasing production capacity and discontinuation due to business strategy. On the other hand, when the prices were set unattainably low without considerable margins, drug suppliers would certainly be reluctant to supply after having delivered the agreed quantity of the contract[31,55].
Based on the results of this study and the specific situation of drug shortage in China, we propose the following recommendations to solve or mitigate the drug shortage problem, including; strengthening pharmaceutical production and quality management. A real-time and sensitive monitoring system should be established to keep track of the production and supply of raw materials to warn and prevent disruption of drug production and pharmaceutical companies should be encouraged to explore stricter internal quality management systems on basis of GMP to avoid sudden drug shortages due to quality problem. Ensuring drug distribution a modern logistics system should be constructed to improve the concentration of the pharmaceutical market and enhance the stability of drug supply. A central pharmacy could be established to centralize the procurement of drugs with low clinical demand. Optimizing drug policy the taking volumebased procurement for example, terms of responsibility for the manufacturer’s inability to supply drugs should be clarified in the procurement contract. And a scientific and reasonable pricing model should be explored considering drug quality and supply sustainability to avoid drug shortage caused by low price.
The data used in this study based on multiple sources, including medical institutions at all levels, administrative departments, and pharmaceutical companies, so our result could provide a rather realistic picture of the drug shortage in Sichuan Province. But two main limitations of this study should be noted. First, the data in this study was extracted from 1st January, 2018 to 30th May, 2021, when the Coronavirus Disease-2019 (COVID-19) pandemic had a significant impact on global drug supply chains. But due to the availability of data, we could not analyse the confounding effect of COVID-19. Second, most of the results of this study were descriptive and no quantitative analysis of the clinical outcomes, economic losses and social impact of drug shortages was performed, so further research was needed to assess the impact.
In conclusion, this study has fully described and analysed the drug shortage situation in Sichuan Province based on multi-source reporting data. A plethora of drugs experienced a shortage in Sichuan Province, covering nearly all the ATC classification. A large portion of the drugs were oral, but the ASF of injectable was the highest. The incidence of drug shortage on the EDL and NEML was significantly higher than that of the PDL and NMIDL. Drug shortage affected the entire drug supply chain, including production, delivery and utilization. To address this issue, it was imperative to foster enhanced collaboration among all stakeholders to mitigate and resolve the drug shortage problem.
Acknowledgements:
We gratefully acknowledged the Health Commission of Sichuan Province for providing the data analysed in this study.
Funding:
This study was funded by the Medical Interdisciplinary Key Project of Sichuan University (Grant No: 202207).
Conflict of interests:
The authors declared no conflict of interests.
References
- Ravela R, Lyles A, Airaksinen M. National and transnational drug shortages: A quantitative descriptive study of public registers in Europe and the USA. BMC Health Serv Res 2022;22(1):940.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Medicines shortages: Global approaches to addressing shortages of essential medicines in health systems. WHO Drug Info 2016;30(2):180-5.
- World Health Assembly. Addressing the global problem shortage of medicines shortages: Draft resolution proposed by the delegations of Colombia, India, Kenya, South Africa, and United States of America. World Health Organization 2016.
- Tan YX, Moles RJ, Chaar BB. Medicine shortages in Australia: Causes, impact and management strategies in the community setting. Int J Clin Pharm 2016;38:1133-41.
[Crossref] [Google Scholar] [PubMed]
- Lukmanji S, Sauro KM, Josephson CB, Altura KC, Wiebe S, Jetté N. A longitudinal cohort study on the impact of the clobazam shortage on patients with epilepsy. Epilepsia 2018;59(2):468-78.
[Crossref] [Google Scholar] [PubMed]
- Phuong JM, Penm J, Chaar B, Oldfield LD, Moles R. The impacts of medication shortages on patient outcomes: A scoping review. PloS One 2019;14(5):e0215837.
[Crossref] [Google Scholar] [PubMed]
- Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA 2017;317(14):1433-42.
[Crossref] [Google Scholar] [PubMed]
- Hedlund NG, Isgor Z, Zwanziger J, Rondelli D, Crawford SY, Hynes DM, et al. Drug shortage impacts patient receipt of induction treatment. Health Serv Res 2018;53(6):5078-105.
[Crossref] [Google Scholar] [PubMed]
- Becker DJ, Talwar S, Levy BP, Thorn M, Roitman J, Blum RH, et al. Impact of oncology drug shortages on patient therapy: Unplanned treatment changes. J Oncol Pract 2013;9(4):e122-8.
[Crossref] [Google Scholar] [PubMed]
- Gatesman ML, Smith TJ. The shortage of essential chemotherapy drugs in the United States. N Engl J Med 2011;365(18):1653-5.
[Crossref] [Google Scholar] [PubMed]
- Poulsen JH, Nørgaard LS, Dieckmann P, Clemmensen MH. Time spent by hospital personnel on drug changes: A time and motion study from an in- and outpatient hospital setting. Plos One 2021;16(2):e0247499.
[Crossref] [Google Scholar] [PubMed]
- Miljković N, Batista A, Polidori P, Kohl S, Horák P. Results of EAHP’s 2019 medicines shortages survey. Eur J Hosp Pharm 2020;27(4):202-8.
[Crossref] [Google Scholar] [PubMed]
- Drug shortages: Root causes and potential solutions. Food drug administration; 2020.
- Claus B, Pauwels K, Baert M, Depoorter J, de Weerdt E, Boussery K, et al. Drug shortages in the hospital: Management, causes and budget impact. J Pharm Bel 2015(1):24-34.
[Google Scholar] [PubMed]
- Beijing: Policy interpretation of implementation opinions on reforming and improving the shortage drug supply security mechanism. The National Health and Family Planning Commission of the People's Republic of China; 2017.
- Beijing: Further work to improve the supply and price stabilization of drugs in shortage. The General Office of the State Council of the People's Republic of China; 2019.
- Beijing: Policy interpretation of government notification on issuance of the national list of drugs in shortage. The National Health Commission of the People's Republic of China; 2020.
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13:31-4.
[Crossref] [Google Scholar] [PubMed]
- Beijing: The National Essential Medicines List. The National Health Commission of the People's Republic of China; 2018.
- The National Healthcare Security Administration. Beijing: The Notice on Printing and Distributing the Medicine List for National Basic Medical Insurance, Industrial Injury Insurance and Maternity Insurance; 2021.
- The National Health Commission of the People's Republic of China. Beijing: The demonstration drugs of directly online purchased emergency (rescue) drugs, paediatric generic drugs obstetrical and gynaecological generic drugs (chemicals and biological products); 2015.
- The National Medical Products Administration. Beijing: Data query: Drugs; 2024.
- Ma LM, Wang H, Zhao Y, Li S, Huang P. Study on the causes and coping strategies of drug shortage in Hubei province. Med Soc 2020;33(8):34-8.
- Yingnan L. Current Situation of Drug Shortage Monitoring and Early Warning. Pharm Chin 2020;15(11):88-91.
- Yang C, Cai W, Li Z, Page AT, Fang Y. The current status and effects of emergency drug shortages in China: Perceptions of emergency department physicians. PLoS One 2018;13(10):e0205238.
[Crossref] [Google Scholar] [PubMed]
- Li X, Lu J, Hu S, Cheng KK, de Maeseneer J, Meng Q, et al. The primary health-care system in China. Lancet 2017;390(10112):2584-94.
[Crossref] [Google Scholar] [PubMed]
- Hernandez I, Hershey TB, Donohue JM. Drug shortages in the United States: Are some prices too low? JAMA 2020;323(9):819-20.
- State Administration for Market Regulation. Beijing: Measures for the supervision and administration of Drug Production; 2020.
- Patel R, Samiee-Zafarghandy S, Ziesenitz V, Fox ER, van den Anker J, Ong H, et al. US drug shortages compared to the World Health Organization’s model list of essential medicines for Children: A cross-sectional study. Am J Health Syst Pharm 2022;79(22):2012-7.
[Crossref] [Google Scholar] [PubMed]
- Benhabib A, Ioughlissen S, Ratignier-Carbonneil C, Maison P. The French reporting system for drug shortages: Description and trends from 2012 to 2018: An observational retrospective study. BMJ Open 2020;10(3):e034033.
[Crossref] [Google Scholar] [PubMed]
- Pauwels K, Huys I, Casteels M, Simoens S. Drug shortages in European countries: A trade-off between market attractiveness and cost containment? BMC Health Serv Res 2014;14:1-9.
[Crossref] [Google Scholar] [PubMed]
- Shukar S, Zahoor F, Hayat K, Saeed A, Gillani AH, Omer S, et al. Drug shortage: Causes, impact, and mitigation strategies. Front Pharmacol 2021;12:693426.
[Crossref] [Google Scholar] [PubMed]
- Izutsu KI, Abe Y, Kurita M, Yoshida H. Shortages of prescription drugs due to compliance and quality issues in Japan. Yakugaku Zasshi 2023;143(2):139-52.
[Crossref] [Google Scholar] [PubMed]
- Fox ER, Sweet BV, Jensen V. Drug shortages: A complex health care crisis. Mayo Clin Proc 2014;89(3):361-73.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Guh DP, Sun H, Lynd LD, Hollis A, Grootendorst P, et al. Factors associated with drug shortages in Canada: A retrospective cohort study. CMAJ Open 2020;8(3):e535-44.
[Crossref] [Google Scholar] [PubMed]
- Sarnola K, Kari H, Koskinen H. Medicine shortages: Product life cycle phases and characteristics of medicines in short supply-A register study. Front Pharmacol 2022;13:943249. 2022;13:943249.
[Crossref] [Google Scholar] [PubMed]
- Omer S, Pan M, Ali S, Shukar S, Fang Y, Yang C. Perceptions of pharmacists towards drug shortages in the healthcare system of Pakistan and its impact on patient care: Findings from a cross-sectional survey. BMJ Open 2021;11(12):e050196.
[Crossref] [Google Scholar] [PubMed]
- Reed BN, Fox ER, Konig M, Jackevicius CA, Masoudi FA, Rabinstein AA, et al. The impact of drug shortages on patients with cardiovascular disease: Causes, consequences, and a call to action. Am Heart J 2016;175:130-41.
[Crossref] [Google Scholar] [PubMed]
- American Society of Health-System Pharmacists. Drug shortages statistics. University of Utah Drug Information Service; 2018.
- Li X, Wu C, Lu J, Chen B, Li Y, Yang Y, et al. Cardiovascular risk factors in China: A nationwide population-based cohort study. Lancet Public Health 2020;5(12):e672-81.
[Crossref] [Google Scholar] [PubMed]
- Report on cardiovascular health and diseases in China 2021: An updated summary. Biomed Environ Sci 2022;35(7):573-603.
[Crossref] [Google Scholar] [PubMed]
- Li J, Sun F, Guo Y, Fan H. High-salt diet gets involved in gastrointestinal diseases through the reshaping of gastroenterological milieu. Digestion 2019;99(4):267-74.
[Crossref] [Google Scholar] [PubMed]
- Yang C, Wu L, Cai W, Zhu W, Shen Q, Li Z, et al. Current situation, determinants, and solutions to drug shortages in Shaanxi Province, China: A qualitative study. PloS One 2016;11(10):e0165183.
[Crossref] [Google Scholar] [PubMed]
- Song Y, Li J, Zhao F, Jin P. Drug shortages in China: A cross-sectional study. BMC Health Serv Res 2023;23(1):438.
- Lin MP, Vargas-Torres C, Shin-Kim J, Tin J, Fox E. Nearly all thirty most frequently used emergency department drugs experienced shortages from 2006-2019. Am J Emerg Med 2022;53:135-9.
[Crossref] [Google Scholar] [PubMed]
- Mazer-Amirshahi M, Pourmand A, Singer S, Pines JM, van den Anker J. Critical drug shortages: Implications for emergency medicine. Acad Emerg Med 2014;21(6):704-11.
[Crossref] [Google Scholar] [PubMed]
- Donnelly KA, Zocchi MS, Katy TA, Fox ER, Pines JM, van den Anker JN, et al. Prescription drug shortages: Pediatric emergency and critical care medications. Pediatr Emerg Care 2021;37(11):e726-31.
[Crossref] [Google Scholar] [PubMed]
- Wu W, Tang Z, Chen J, Gao Y. Pediatric drug development in China: Reforms and challenges. Pharmacol Res 2019;148:104412.
[Crossref] [Google Scholar] [PubMed]
- Nixon HC. Drug shortages in obstetrics. Curr Anesthesiol Rep 2021;11:28-36.
- Bogaert P, Bochenek T, Prokop A, Pilc A. A qualitative approach to a better understanding of the problems underlying drug shortages, as viewed from Belgian, French and the European Union’s perspectives. PloS One 2015;10(5):e0125691.
[Crossref] [Google Scholar] [PubMed]
- Xing Q, Tang W, Li M, Li S. Has the volume-based drug purchasing approach achieved equilibrium among various stakeholders? Evidence from China. Int J Environ Res Public Health 2022;19(7):4285.
[Crossref] [Google Scholar] [PubMed]
- Chabner BA. Drug shortages: A critical challenge for the generic drug market. N Engl J Med 2011;365:2147-9.
[Crossref] [Google Scholar] [PubMed]
- Woodcock J, Wosinska M. Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther 2013;93(2):170-6.
[Crossref] [Google Scholar] [PubMed]
- Fox ER, Birt A, James KB, Kokko H, Salverson S, Soflin DL. ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health Syst Pharm 2009;66(15):1399-406.
- Shuman A, Unguru Y. Drug shortages: The view across an ocean. Oncologist 2020;25(4):274-6.
[Crossref] [Google Scholar] [PubMed]
 Other causes;
Other causes;  Distribution problems;
Distribution problems; 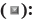 Manufacturing
problems and
Manufacturing
problems and  Discontinuation
Discontinuation



