- Corresponding Author:
- N. Udupa
Manipal College of Pharmaceutical Sciences, MAHE, Manipal-576 104, India.
E-mail: n.udupa@manipal.edu
| Date of Submission : | 07 January 2005 |
| Date of Revision : | 28 January 2006 |
| Date of Acceptance : | 27 October 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 680-682 |
Abstract
Valdecoxib is a nonsteroidal antiinflammatory drug, and it is listed in class 2 of biopharmaceutic classification of drugs. Valdecoxib is a poorly water-soluble and highly permeable drug. In the present study a new dissolution medium was developed, as there is no official dissolution medium available in the literature. The composition of the dissolution medium was selected on the basis of solubility data at 37°. Solubility data revealed that addition of surfactant may be suitable as dissolution medium. The concentration of 0.6% w/v sodium lauryl sulphate in water could be a suitable dissolution medium. The discriminating power of the selected dissolution medium (0.6% sodium lauryl sulphate in water) relative to the other dissolution mediums was evaluated. The selected dissolution medium was used for the evaluation of valdecoxib tablets.
Valdecoxib (VAL) is a new nonsteroidal antiinflammatory drug (NSAID) [1]. It is a selective inhibitor of the cyclooxygenase-2 (COX-2) and exhibits many of the pharmacological actions of prototypical NSAIDs, including antiinflammatory, analgesic and antipyretic activity [2]. It is not official in any pharmacopoeia. The testing of pharmaceutical dosage forms for in vitro drug release and dissolution characteristics is very important for ensuring batch-to-batch quality control and to optimize formulations during drug development. Drugs that are practically insoluble (less than 0.01%) need special attention during dissolution testing for proper in vitro-in vivo correlation. Since the dissolution may be a rate limiting step in the in vivo absorption process, there is a need for developing an appropriate dissolution medium [3].
Approaches usually used in the design of dissolution media for poorly water-soluble drugs include increasing the volume of the aqueous sink or removing the dissolved drug, solubilization of the drug by co-solvents (up to 40%), or by anionic or non-ionic surfactants (in post-micellar concentrations) or alteration of pH to enhance the solubility of insoluble drug molecules [4,5]. The last two approaches seem less cumbersome and have been more widely employed in pharmacopoeial dissolution tests [6].
In the present investigation, aqueous solubility of VAL in media containing co-solvents or surfactants was assessed to prepare a dissolution system which satisfies sink conditions for testing VAL formulations. The discriminating power of the selected dissolution medium was evaluated using prepared and commercial formulations.
VAL was a gift sample from M/s IPCA Laboratories Ltd., Mumbai. Two brands of VAL tablets Valus (Glenmark Pharmaceuticals Ltd., Mumbai) and Revaldo-20 (Bangalore Pharmaceutical Research Laboratories, Bangalore) were purchased from the local market. Sodium lauryl sulphate, Tween 80 and ethanol were purchased from Qualigens Fine Chemicals, Mumbai. All other materials were of analytical reagent grade.
The apparent solubility of VAL in water or in presence of co-solvent or surfactant in water was determined at 37°. VAL (50 mg) was added to 50 ml of water in a conical flask with Teflon-lined screw caps. The conical flasks were kept in a shaker incubator maintained at 37±0.5° for 24 h. After shaking, the flasks were kept in an incubator at 37±0.5° for equilibration for 12 h. Then the solution was filtered through 0.45 μ membrane filter, and the filtrate was assayed spectrophotometrically at 239 nm against two respective blank solutions.
Dissolution experiments were performed using USP standard dissolution apparatus II (M/s Electrolab, Mumbai) at 37±0.5° at a paddle speed of 75 rpm. The dissolution medium was 900 ml of either water or a mixture of water and sodium lauryl sulphate (SLS) solution, selected on the basis of solubility data obtained from the experiments using 0.2, 0.5, 1, 1.5 or 2% w/v SLS in water. These mediums were also used to test the dissolution of bulk powder samples (100 mg, particle size < 200 μm) of VAL. Samples (5 ml) of dissolution medium were withdrawn at different time intervals and filtered through 0.22 μm millipore filter. Same volume of fresh dissolution medium, maintained at 37±0.5°, was added to maintain constant volume. The dissolution medium was analyzed for VAL using UV method as described above. Results presented are the average of three experiments.
A batch of 20 tablets of VAL was procured for comparative studies of two different brands, Valus and Revaldo-20. The dissolution experiments for commercial formulations of VAL were performed using above in vitro dissolution conditions by selecting 0.6% w/v SLS in water as dissolution medium.
In this study, solubility data was used as a basis for the development of a dissolution medium for VAL. Since VAL is poorly soluble in water, solubility determination was carried out using 0.6, 0.8, 1.0, 1.5 and 2.0% w/v of SLS in water; phosphate buffer pH 7.4, 0.5 and 1.0% v/v Tween in water; 5 and 10% methanol in water. The apparent solubility of VAL in different mediums is given in the Table 1.
| Media | Conc. (µg/ml) |
|---|---|
| Water | 113±5.5 |
| 0.1N HCl | 251±4.0 |
| 0.6% w/v SLS | 517±5.1 |
| 0.8% w/v SLS | 719±8.3 |
| 1.0% w/v SLS | 798±6.1 |
| 1.5% w/v SLS | 1331±7.2 |
| 2.0% w/v SLS | 1471±6.2 |
| pH 7.4 | 200±1.4 |
| 0.5% v/v Tween 80 in water | 302±2.6 |
| 1% v/v Tween 80 in water | 427±3.6 |
| 5% v/v Methanol in water | 208±3.4 |
| 10% v/v Methanol in water | 231±4.1 |
Apparent solubility of VAL (valdecoxib) in various dissolution medium at 37° is given, and the concentration is expressed as micrograms per millilitres.
Table 1: Solubility Studies of Val
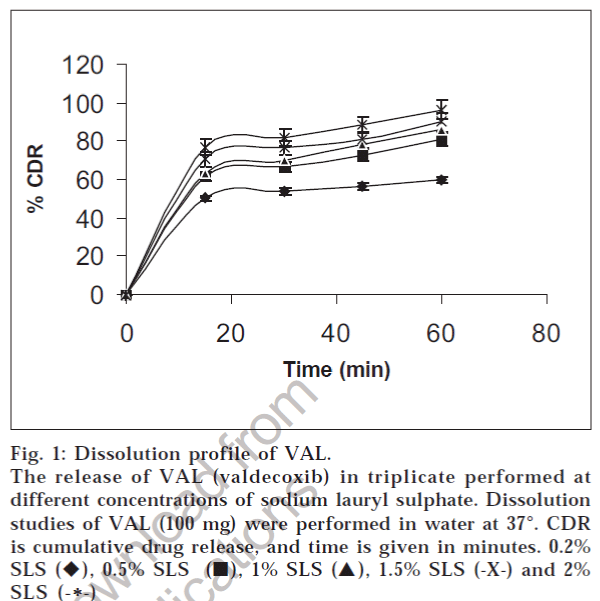
The comparative study of the dissolution rate of pure drug in water and in different ratios of SLS (0.25, 0.5, 1, 1.5, 2% w/v) containing water was carried out to justify the inclusion of SLS in fig.1. The results indicated that dissolution rate of VAL increased with increase in SLS concentration of dissolution medium, and maximum dissolution was found in water containing 2% w/v SLS. Addition of surfactant to the dissolution medium improves the dissolution of pure drug by facilitating the drug release process at the solid / liquid interface and micelle solubilization in the bulk7. However, 0.6% w/v SLS will be sufficient as dissolution medium which has discriminating power when compared to lower concentrations. The solubility of a drug can be enhanced by ensuring that the surfactant concentration is at least above the critical micelle concentration (CMC). The CMC will depend upon the surfactant itself and the ionic strength of the media. The amount of surfactant needed depends on the CMC and the degree to which the compound partitions into the surface micelles [8] . It may be the reason for the fact that increase in concentration of SLS above 2.0% SLS did not exhibit any increase in the amount of VAL dissolved. The apparent solubility of SLS was found to be higher when compared to Tween 80. It may be due to the ionization effect of SLS, a cationic surfactant [9].
Fig. 1: Dissolution profile of VAL. The release of VAL (valdecoxib) in triplicate performed at different concentrations of sodium lauryl sulphate. Dissolution studies of VAL (100 mg) were performed in water at 37°. CDR is cumulative drug release, and time is given in minutes. 0.2% SLS (♦), 0.5% SLS (?), 1% SLS (?), 1.5% SLS (-X-) and 2% SLS (-*-)
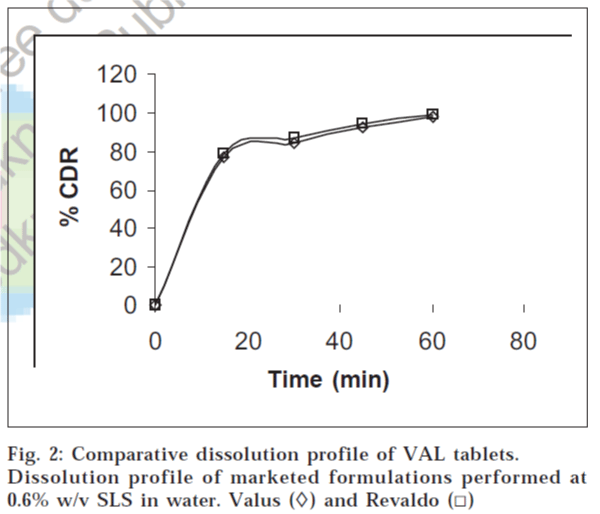
The performance of the selected dissolution medium (900 ml of 0.6% w/v SLS in water) was confirmed by conducting dissolution experiments of commercial formulations, and the results are shown in fig. 2. The release rate of VAL from commercial formulations was determined. It was found that more than 85% of VAL was released from the formulations within 45 min under test conditions. The results of the present study clearly indicate that 0.6% w/v SLS in water, as dissolution medium, was suitable for routine in vitro dissolution testing of conventional VAL formulations.
References
- Kurumbali, R.G., Stevens, A.M., Gierse, J.K., McDonald, J.J., Stegman, R.A., Pak, J.Y., Gildehaus, D., Miyashiro, J.M., Penning, T.D., Seibert, K., Isakson, P.C. and Stalling, W.C., Nature, 1996, 384, 644.
- Peterson, W.L. and Cryer, B., J. Amer. Med. Assn., 1999, 282, 1961
- Gander, B., Ventourase, K., Gurny, R. and Doelkar, E., Int. J. Pharm., 1985, 27, 117.
- Gibladi, M. and Feldman, S., J. Pharm. Sci., 1967, 56, 1238.
- Shah, V.P., Konecny, J.J., Everett, R.L., McCullough, B. and Skelly, J.P., Pharm. Res., 1989, 6, 612.
- United States of Pharmacopoeia 23, US Pharmacopoeia Convention, 1995, 267.
- Abdou, H.M., Hanna, S. and Mohammad, N., In: Gennaro, A.R., Eds., Remington: The Science and Practice of Pharmacy, Vol.1, 20th Edn., Lippncott Williams and Wilkins, Baltimore, Maryland, 2000, 655.
- Schott, H., Kwan, L.C. and Feldman, S., J. Pharm. Sci., 1982, 71, 1038.
- Babu, M.M.G.V., Shankar, G.V., Shankar, H.K., Seshayana, A., Kumar,K.N. and Murthy, R.K.V., Indian J. Pharm. Sci., 2002, 588.