N. B. Dobaria, S. G. Shah and S. J. Rajput*
Pharmaceutical quality assurance laboratory, Pharmacy department, Faculty of technology and engineering, The m. S. University of baroda, kalabhavan, vadodara-390 001, India
- *Corresponding Author:
- S. J. Rajput
Pharmaceutical quality assurance laboratory, Pharmacy department, Faculty of technology and engineering, The m. S. University of baroda, kalabhavan, vadodara-390 001, India
E-mail: sjrajput@rediffmail.com
| Date of Submission | 06 May 2005 |
| Date of Revision | 28 November 2005 |
| Date of Acceptance | 12 August 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 562-565 |
Abstract
A new plastic membrane ion-selective electrode for determination of metformin hydrochloride has been prepared, after forming the ion pair of metformin hydrochloride with sodium tetraphenylborate. Dioctyl phthalate and sodium tetraphenylborate were used as plasticizer and counter ion respectively. The electrode exhibited a linear potential response in the concentration range 1 × 10-5 M–1 × 10-1 M with slope of 42.0±0.82 mv per decade. The electrode has a rapid response time (45 s), shorter conditioning time (2 h) and a lower limit of detection (1 × 10-5 M). The electrode showed high selectivity for metformin hydrochloride with respect to some common ions, excipients and other drugs present as combined dosage formulations. Stability studies of ion-selective membrane were carried out by differential scanning calorimetry. The electrode can successfully be applied for the analysis of metformin hydrochloride in pure solution, pharmaceutical preparations, biological fluid and in presence of its degraded products.
Metformin (Met), 1,1-diethylbiguanide hydrochloride, is an anti-hyperglycaemic agent which inhibits glucogenesis and increases insulin level. It also decreases plasma glucose level by reducing absorption of glucose from the intestine [1]. It is official in IP [2] and BP [3]. Several methods have been reported for the determination of metformin hydrochloride (MetHCl) in pharmaceutical preparations, including spectrophotometry [3], high performance liquid chromatography (HPLC) [4-5], gas chromatography [6] and liquid chromatography-mass spectroscopy (LC-MS) [7]. Coloured and turbid solutions interfere with the measurement of the absorbance in spectrophotometric method. Tedious sample preparation is often required in the chromatographic methods. Ion-selective electrodes have been considered as novel technique for the analysis of drugs due to the larger concentration range of analyte determined, reasonable selectivity, simple operation, precision and accuracy of analytical information [8-13].
Materials and Methods
All the reagents and solvents used were of analytical grade, and double-distilled water was used throughout the experiments. MetHCl was obtained as a gift sample from Sun Pharmaceutical Industries Ltd. (Silvassa), whereas its tablet formulations were procured from the local market. Silicotungstic acid and sodium tetraphenylborate (NaTPB) were obtained from Himedia, Mumbai; sodium phosphotungstate and polyvinyl chloride (PVC) from National Chemicals, Vadodara; dibutyl phthalate (DBP) and dioctyl phthalate (DOP) from Suvidhinath Laboratory, Vadodara; bis (2-ethylhexyl) adipate from S. D. Fine-Chem, Mumbai; and tetrahydrofuran (THF) from Qualigens Fine Chem., Mumbai.
Working standard solutions of MetHCl were prepared by successive dilutions of a 0.1 M stock solution, which was prepared by dissolving 1.656 g MetHCL in 100 ml of double-distilled water.
Preparation of Met-TPB ion pair
The metformin tetraphenylborate (Met-TPB) was used as the electroactive material in the membrane. The Met-TPB ion pair was prepared by mixing 100 ml of 1 × 10-2 M aqueous MetHCl solution with equimolar amounts of sodium tetraphenylborate. The resulting precipitate was filtered out, washed thoroughly with double-distilled water until free from chloride and dried at room temperature. The composition of the Met-TPB ion pair was confirmed by elemental analysis as 1:1. The calculated percentages of C, H and N were 76.71%, 7.30% and 15.98%, while the found percentages were 76.32%, 7.63% and 15.19% respectively.
Preparation of the electrode
The electrode membrane was prepared by mixing the ion pair formed, plasticizer and a polymeric support. The weight ratio of polymer to plasticizer was 1:2. The membranes were prepared by dissolving the required amount of the ion pair, PVC and DBP in about 5 ml of THF. The solution mixture was poured into a glass Petri dish (5 cm in diameter) and was allowed to stand overnight to dry at room temperature. Thickness of membrane was maintained between 18 and 20 μm. The 15 mm diameter disc was cut from the prepared membrane and glued using Teflon tape to the polished end of a plastic tube. The electrode body was filled with a solution of 1×10-2 M MetHCl as internal reference solution. The electrode was preconditioned by soaking in 1×10-3 M MetHCl for 1 h but taken out from the solution if not in use.
Measurement of electromotive force (EMF)
The EMF measurements were carried out at room temperature with a Li 120 digital pH/mv meter (Elico Pvt. Ltd., Hyderabad). The newly developed metformin-selective PVC membrane electrode and saturated calomel electrode (SCE) were used as indicator and reference electrode respectively. The electrochemical cell was - SCE/test solution/MetHCl-SE
Preparation of calibration curve
A series of standard solutions (1×10-5 M-1×10-1 M) of MetHCl were prepared by successive dilution of a 0.1 M stock solution. The potential responses of electrode were measured and plotted against different concentrations of metformin hydrochloride solutions. The electrode exhibited a linear potential response in the concentration range of 1×10-5 M-1×10-1 M with slope 42.0±0.82 mv per decade and correlation coefficient 0.9922. The limit of quantification was 1×10-1 M. The electrode required a preconditioning time of 2 h with a response time of 45 s.
Determination of metformin hydrochloride in pharmaceutical formulations
Twenty tablets were accurately weighed and powered in a mortar. Tablet powder equivalent to 500 mg MetHCl was dissolved in 100 ml of double-distilled water. The metformin ion-selective electrode and reference electrode were immersed into test solution. The mv reading was recorded. The amount of MetHCl in tablet was determined from the calibration curve.
Determination of metformin hydrochloride in spiked human serum sample
Different amounts of MetHCl and 0.1 ml of human serum were transferred to 100 ml volumetric flask and diluted up to the mark with double-distilled water. The solution was transferred to 100 ml beaker and subjected to potentiometric determination of MetHCl by standard addition method. The mean values of recoveries and relative standard deviation were found to be 100.9 and 3.0% respectively.
Determination of metformin hydrochloride in presence of its degraded products
Metformin hydrochloride was subjected to stress degradation using 0.1 M HCl and 30% (v/v) H2O2as per ICH guidelines. Different amounts of degraded MetHCl were transferred to 100 ml volumetric flask, diluted to the mark with double-distilled water, transferred to 100 ml beaker and subjected to potentiometric determination of MetHCl by standard addition method. The mean values of recoveries and standard deviation were found to be 99.96 and 0.54% respectively.
Results and Discussion
Two ion pairing agents, silicotungstic acid and dibutyl phthalate, formed ion pair complex with Met but due to insolubility of Met-silicotungstate ion pair in THF, the plastic membrane could not be prepared, and Met-dibutyl phthalate ion pair was used to prepare the membrane.
The membrane composition was studied by varying the percentages (w/w) of the ion pair, polyvinyl chloride (PVC) and dibutyl phthalate (DBP) until the optimum composition exhibiting the best linear responses was obtained. This composition is shown in Table 1. The effect of the different plasticizers like dibutyl phthalate, dioctyl phthalate and bis (2-ethylhexyl) adipate on the Met-TPB electrode was also studied (fig. 1). The use of dibutyl phthalate as plasticizer gave more stable and linear potential response in comparison to dioctyl phthalate and bis (2-ethylhexyl) adipate. The electrode response with membrane plasticized with dioctyl phthalate showed large fluctuations in potential measurement, whereas the linearity range was less if bis (2-ethylhexyl) adipate was used as plasticizer.
| Electrode composition, % (w/w) | ||||
|---|---|---|---|---|
| Membrane | Ion pair | PVC | DBP | Correlation coefficient |
| I | 0.8 | 34.6 | 64.6 | 0.9844 |
| I I | 1 | 34.5 | 64.5 | 0.9922 |
| I II | 3 | 32.5 | 62.5 | 0.9691 |
| I V | 5 | 30 | 60 | 0.9555 |
Membrane was finally used for the construction of electrode. The ion pairing agent was sodium tetraphenyl borate, PVC is polyvinyl chloride and DPP is diphenyl phthalate, used as plasticizer
Table 1: Composition of metformin ion selective plastic membrane electrode
Lifespan of electrode depends upon the stability of ion pairing complex in ion-selective membrane. A stability study of ion-selective membrane was carried out using DSC. Thermograms (fig. 2) of ion-selective membrane at different time intervals and before and after conditioning the membranes were obtained. The curves B, C and D showed that there was no significant difference in the membranes up to 2 w in terms of the thermogram parameters, but after 2 w of storage (curves E and F), membrane characteristics were significantly changed. These studies revealed that ion-selective membrane was stable for 2 w.
The response time of electrode was tested at 1×10-1 M-1×10-5 M of MetHCl solution, and response time was 45 s. The stability of electrode response was monitored continuously for a 1×10-2 M MetHCl solution. The measurements were repeated six times for a series of MetHCl solutions (1×10-1M-1×10-5 M) to assess the repeatability of the measurements. The relative standard deviations for six replicate measurements obtained were 0.76%.
The selectivity of electrode towards some common ions was investigated by separate solution method [14] and calculated from the equation:
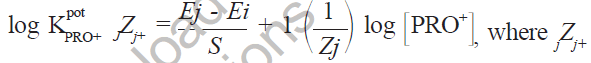
is the interfering ion, JZj is the charge of interfering ion, Ej and Ei are the electrode potentials of 1.0×10-3 M solution of the primary ion (PRO+) and interfering ion (JZj) respectively, S is the slope of the calibration graph for PRO+. Selectivity coefficients for Na+, Ca++, K+ and Mg++ ions were 0.7992, 0.2460, 1.095 and 0.5140 respectively.
Drugs like glimipremide, glypizide, pioglitazone and glibenclamide did not interfere in the analysis, so MetHCl could be estimated in the combination formulations with these drugs. The results are shown in Table 2.
| Brand name | Content | mv* | Label claim, mg | Obtained amount | % recovery |
|---|---|---|---|---|---|
| Piosafe MF 15/500 Glipimet fort 5/500 Glimeprex MF 1/500 Glucored fort 5/500 | Pioglitazone and metformin HCl Glipizide and metformin HCl Glimepride and metformin HCl Glibenclamide and metformin HCl | 96.6 | 500 | 506.72 | 101.34 |
| 96.2 | 500 | 495.73 | 99.14 | ||
| 96.8 | 500 | 512.31 | 102.46 | ||
| 96.6 | 500 | 506.72 | 101.34 | ||
| *Average of five determinations | |||||
*Average of five determinations
Table 2: Analysis of marketed formulations using methcl ion selective electrode
The developed novel ion-selective electrode was successful in analyzing MetHCl in pure solutions and in pharmaceutical preparations, and the results are compared with the pharmacopoeial method. The mean value (490 mg/tablet) and the relative standard deviation (1.8%, n = 5) obtained by using ion-selective membrane electrode method were in good agreement with the value of 496 mg/tablet obtained by using pharmacopoeial method. The recovery study was also done, and the mean values of recoveries and relative standard deviation were found to be 101.32 and 0.84% respectively.
The proposed ion-selective electrode is simple to use, highly selective and inexpensive. The electrode gives quick response with high accuracy and precision and does not require any sample treatment for analyzing drug in plasma.
Acknowledgements
We are thankful to Dr. Surekha Devi, Head, Chemistry Department; and Dr. N. S. S. Murthy, Metallurgy Department, of The M. S. University of Baroda for providing facilities for elemental analysis and thickness measurement of the membrane respectively. We are grateful to M/s Sun Pharmaceuticals Ltd. (Silvassa) for their generous gift of metformin hydrochloride, and to Mr. V. Sutariya for help in taking DSC.
References
- Davis, N.S. and Granner, D.K., In; Goodman and Gillman Eds., ThePharma cological Basis of Therapeutics, 9th Edn, McGraw-Hill, NY, 1996, 1507.
- Indian Pharmacopoeia, 4th Edn., Vol.1, The Controller of Publications, New Delhi, 1996, 469.
- The British Pharmacopoeia, Vol.1, Her Majesty’s Stationery Office, Norwich, 1993, 1013.
- Charles, B.G., Jacpsen, N. W. and Ravenscroft, P., J. Clin. Chem., 1981, 27, 434.
- Bonfigil, A.R., Manfrini, S. and Testa, R., Drug Monitor, 1999, 21, 332.
- Brohon, J. and Jacobsen, N.W., J. Clin. Chem., 1978, 146, 148.
- Wang, Y., Tang, Y., and Bai, X., J. Chromatogr. B. Anal.Techno. Biomed., Life Sci., 2004, 808, 215.
- Zayed, S.I.M., Anal. Sci., 2004, 20, 1043.
- Lenik, J., Dumkiewicz, R. and Wardak, C.,Acta. Pol. Pharm.,2002, 59, 171.
- Shamshipur, M., Jalal, F. and Haghgoo, S., J. Pharm. Biomed.Anal., 2002, 27, 867.
- Erdem, E., Ozkan, D., Kerman, K. and Meril, B., Turk J. Chem.,2000, 20, 353.
- Shoukry, A.F., Abdel-Ghani, N.T., Issa, Y.M. and Ahmed, H.M.,Electroanalysis, 1999, 11, 443.
- Stefan, R.I. and Aboul-Enein, H.Y., Accred. Qual. Assur., 1998,3, 194.
- Ren. K. and Fresenius, M., J. Anal. Chem., 1999, 365, 389.