- *Corresponding Author:
- T. R. Saini
Industrial Pharmacy Research Lab, Department of Pharmacy
Shri G. S. Institute of Technology and Science, Indore-452003, India
E-mail: proftsaini@gmail.com
| Date of Submission | 03 April 2017 |
| Date of Revision | 25 December 2017 |
| Date of Acceptance | 18 July 2018 |
| Indian J Pharm Sci 2018;80(5):813-819 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present investigations report the application of SABER® technology in the development of a one-month depot injection of iloperidone. SABER® is a new in situ gel formulation technology for sustained release drug delivery, which precludes the use of large amounts of toxic organic solvents in the formulation. Traditionally in the in situ gel forming technology-based depot injections, expensive polymers such as polylactic acid and poly(lactic-co-glycolic acid), and a class 2 solvent like N-methyl-2-pyrrolidone have been used to control the rate of drug release and dissolve the drug and polymer, respectively. In the present investigations, however, no such release retarding polymers were used, moreover, the formulation was developed using a limited amount of dimethylsulphoxide, a class 3 solvent. Formulation variables were optimized by D-optimal mixture design to obtain the depot formulation with a consistent drug release for one month. The composition of sucrose acetate isobutyrate, and dimethylsulphoxide were taken as input variables and cumulative drug release at various time points as response variables. The optimized formulation contained 81.718 % sucrose acetate isobutyrate and 18.282 % dimethylsulphoxide and produced a consistent drug release profile with 85.71 % cumulative drug release in 30 days without any significant burst release.
Keywords
Sucrose acetate isobutyrate, in situ gel forming depot injection, iloperidone, dimethylsulphoxide, D-optimal mixture design
Iloperidone is a new atypical antipsychotic drug approved by USFDA in 2009 for the treatment of schizophrenia [1]. It is effective against positive as well as negative symptoms of schizophrenia and has many specific advantages over other atypical antipsychotics. It has a low tendency to induce extrapyramidal symptoms and is well tolerated [2]. Presently it is available as a tablet dosage form in 1-12 mg strengths with a maximum daily dose of 24 mg. Its elimination half-life is 18 h in case of extensive CYP2D6 metabolizers and 33 h in poor CYP2D6 metabolizers [3]. However, despite having a half-life of 18-33 h, it needs to be administered twice a day to minimize the orthostatic hypotension in the uptitration phase [1]. Now-a-days antipsychotic drug therapy is clinically preferred with depot injections as they ensure uninterrupted and consistent drug delivery for 1-3 mo. Presently no depot formulation of iloperidone is available, though a microcrystals-based depot preparation is under development and a US patent has been granted to Novartis Pharmaceuticals [4].
Oral antipsychotic drug therapy’s major drawback is poor patient compliance and associated increased risk of non-adherence to the dosage regimen [5]. According to one report, 40 % psychotic patients adhere poorly to their medication schedule while 61 % patients develop this problem at some point of time during drug therapy [6]. The above behavior of patients towards oral drug therapy leads to inconsistency in drug administration and relapse of psychosis. Long acting injections overcome the above drawback of oral drug therapy as the direct involvement of patient in daily drug administration at different time intervals is avoided. This eliminates the possibility of interruptions in regular drug administration and therefore considered as an effective and better means of antipsychotic drug therapy with lower relapse rate [7].
Sucrose acetate isobutyrate (SAIB) is a US FDA approved food additive with a safe human daily intake of up to 20 mg/kg [8]. It is extensively metabolized in the body to sucrose and partially acylated sucrose, which are readily absorbed and subsequently eliminated from the body [9]. Its extravascular parenteral administration has been shown to be biocompatible and well tolerated in rats through intramuscular and subcutaneous routes with no signs of serious inflammation or necrosis in histological examination at the site of injection [10,11]. For quite some time, it is being tried in the formulation designing of in situ gel forming depot injections and has been successfully evaluated to provide sustained drug delivery of an anesthetic agent in post-surgical pain management through depot injection [12]. The SAIB-based in situ gel forming depot injections are economical formulations also offering the benefit of ease of manufacturing [13]. The SABER® systems use SAIB along with a solvent and a release modifier for sustained release of therapeutic agents [10]. SAIB is an extremely hydrophobic viscous liquid but it forms a low viscosity fluid when dissolved in some organic solvents [14]. If the solvent is water miscible, the resulting fluid can be used as a vehicle for designing long acting intramuscular or subcutaneous in situ gel forming depot injection. The solvent present in the formulation would eventually diffuse out when it would come in contact with aqueous biological fluid present at the site of injection leaving a highly viscous biodegradable SAIB-drug matrix, which would act as a drug depot for extended drug release in vivo [15,16].
Systematic product development based on quality by design has become a well-established approach in the formulation development [17]. The prime objective of quality by design is to achieve performancebased quality attributes in the product [18]. Several experimental designs are available with their specific applications. Mixture designs are used in the designing of formulations containing multiple excipients and in which the properties of the finished product do not depend on the amount of each excipient present but on their proportions, and the sum total of the proportions of different excipients is unity without any negative fraction [19]. The D-optimal mixture design was employed in the present studies because it involves smaller number of runs and thereby reduces development cost [20].
Most of the reported and marketed in situ gel forming implants employ polylactic acid (PLA), poly(lacticco- glycolic acid (PLGA) and their derivatives as biodegradable release retarding polymers for controlling the drug release from the depot formed in situ [10,11,21,22]. These polymers are, however, quite expensive and add to the cost of the developed depot formulation. These products invariably also use a class 2 organic solvent like N-methyl-2-pyrrolidone (NMP), which is extremely toxic. In the present investigations an attempt was made to develop a long acting depot injection of iloperidone using SAIB as release retarding material, which is much cheaper than PLGA, and dimethylsulphoxide (DMSO) as solvent, which is a class 3 solvent and safer as compared to NMP.
Materials and Methods
SAIB was supplied by M/S Eastman Chemical Company, Kingsport, USA and iloperidone by M/S Sun Pharmaceutical Industries Ltd., Mumbai, India as gift samples. All other chemicals and solvents used were of reagent grade and purchased from market.
Particle size of iloperidone powder:
The average particle size and polydispersity index of the iloperidone drug powder was determined by Malvern Mastersizer 2000 (Malvern, UK) using Millipore water containing 0.02 % Tween 80 as a dispersant.
Solubility determination:
The solubility of iloperidone was determined in purified water, different organic solvents, drug release test medium comprising of phosphate buffered saline (PBS) pH 7.4+0.5 % sodium lauryl sulphate (SLS)+0.05 % sodium azide, and drug release test medium containing DMSO and SAIB in the same ratio as used in the final formulation. An excess quantity of drug was added to each of the above solvents in stoppered glass test tubes, which were kept on a shaker water bath at room temperature for 48 h. The saturated solutions were filtered through 0.45 μm membrane filter and the drug concentration was estimated spectrophotometrically at 276 nm wavelength on a double beam UV/Vis spectrophotometer (UV-1700, Shimadzu, Japan).
Preparation of long acting in situ gel forming depot injection:
Weighed quantity of SAIB was dissolved in a measured volume of ethanol/triacetin/DMSO using a vortex mixer. Iloperidone powder was weighed and added to the SAIB solution and mixed well to get a uniform suspension. Different formulation batches were designed with varied concentrations of SAIB and solvent.
In vitro drug release:
The formulated product was injected into a 15 ml screwcapped plastic tube containing 10 ml drug release test medium, which on coming in contact with aqueous fluid, formed an in situ gel depot matrix. The tubes were placed in an incubator shaker bath maintained at a temperature of 37 ± 1° and operated at 60 ± 5 rpm [23]. At different time intervals, entire 10 ml drug release fluid was pipetted out and replaced with 10 ml fresh drug release test medium. The withdrawn samples were analysed on a double beam UV/Vis spectrophotometer at 276 nm.
Experimental design:
The most important objective of long acting depot injection designing is to achieve the requirement of sustained drug release up to the period of dosing regimen. In the present formulation optimization studies, the upper and lower levels of the input variables were decided on the basis of initial screening trials. A statistical design of experiment (DOE) was planned in which the percent concentration of the formulation excipients, i.e., SAIB and DMSO was selected as input variable and the cumulative percent drug release at various time points was selected as response variable. The optimization was done by D-optimal mixture design using Design Expert software (version 7.1.5, Stat-Ease Inc., Minneapolis, Minnesota, USA).
Results and Discussion
The iloperidone powder was in micronized form with an average particle size of 2.64 μm (surface mean) and 7.7 μm (volume mean) with d50 and d90 values of 6.08 and 14.90 μm, respectively. The solubility of iloperidone was determined in purified water and various other solvents and reported in Table 1. As the solubility of iloperidone in water and PBS (pH 7.4) was poor, 0.5 % SLS was added to PBS as a surfactant to attain sink conditions in drug release study. Because the studies were to be conducted for a long time period, 0.05 % sodium azide was also added in the release test medium as a preservative. Iloperidone was stable in the release test medium for one month.
| Medium | Solubility (mg/ml) |
|---|---|
| Purified water | 0.018 ± 0.0009 |
| DMSO | 21.795 ± 1.068 |
| Triacetin | 110.437 ± 9.156 |
| Ethanol | 4.822 ± 0.564 |
| PBS | 0.005 ± 0.0002 |
| Drug release test medium (PBS+0.5 % SLS) Drug release test medium+excipients in formulation (DMSO and SAIB) |
0.873 ± 0.067 1.073 ± 0.060 |
Mean ± SD, n=3, DMSO is dimethylsulphoxide; PBS is phosphate buffered saline (pH 7.4); SLS is sodium lauryl sulphate and SAIB is sucrose acetate isobutyrate
Table 1: Solubility of iloperidone in different solvent media
SAIB is a very viscous fluid, but when dissolved in even a small amount of certain organic solvents it forms a low viscosity solution. In the present studies, SAIB solutions were prepared in DMSO, triacetin, and ethanol, respectively. The in situ gel forming depot injections were formulated by dispersing iloperidone in each of the above SAIB solutions (IL/IS/PRE1-3). A plain iloperidone dispersion in drug release test medium without SAIB solution was used as the control (IL/IS/PRE4). The composition of formulations designed for pre-optimization studies is shown in Table 2. The final selection of solvent was done on the basis of in vitro drug release from the respective solvent-based formulation as well as their toxicological considerations. The formulated depot injections were subjected to in vitro drug release study for 30 d. The drug release data are recorded in Table 3.
| Formulation batch code | Drug(mg) | SAIB (%) | Ethanol (%) | Triacetin (%) | DMSO (%) |
|---|---|---|---|---|---|
| IL/IS/PRE1 | 30 | 90 | 10 | - | - |
| IL/IS/PRE2 | 30 | 90 | - | 10 | - |
| IL/IS/PRE3 | 30 | 90 | - | - | 10 |
| IL/IS/PRE4 | 30 | - | - | - | - |
Table 2: Composition of pre-optimization batches of in situ gel forming depot injections of iloperidone
As evident from Table 3, the first day in vitro drug release from the designed injection formulations containing ethanol, triacetin, and DMSO as solvent was 21.81, 3.18, and 8.61 %, respectively. The release profile showed almost zero burst release with triacetin, 8.61 % burst release with DMSO, and 21.81 % burst release with ethanol-based formulations. The time taken for 50 % drug release (T50 %) was found to be 5, 25, and 17 d and for 90 % drug release (T90 %) it was calculated to be 27, 47, and 37 d from the formulations containing ethanol, triacetin, and DMSO, respectively. The ethanol-based formulation (IL/IS/PRE1) showed highest burst release and 50 % cumulative drug release in 5 d whereas triacetin-based formulation (IL/IS/ PRE2) showed 50 % drug release in 25 d without any burst release, however, the total drug release from this formulation in 30 d was 52 % only. The DMSO-based formulation (IL/IS/PRE3) on the other hand showed a little burst release and exhibited 50 % drug release in 17 d and 69 % drug release in 30 d. The plain iloperidone drug dispersion in drug release test medium without SAIB and solvent (IL/IS/PRE4) dissolved completely in 1 d indicating that the drug itself cannot provide any sustained release. The sampling time for plain iloperidone was 1, 3, 6, 12, and 24 h. Considering initial burst release and subsequent release patterns, the release profile of DMSO-based formulation was found to be better than ethanol and triacetin-based formulations.
| Formulation batch code |
In vitro cumulative % drug release | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 15 | Day 22 | Day 30 | |
| IL/IS/PRE1 | 21.81 ± 2.25 | 38.17 ± 0.86 | 56.38 ± 3.29 | 72.81 ± 2.66 | 79.42 ± 0.81 | 85.9 ± 2.40 |
| IL/IS/PRE2 | 3.18 ± 0.57 | 13.93 ± 0.61 | 28.96 ± 3.28 | 42.22 ± 2.34 | 46.74 ± 3.07 | 52.13 ± 2.77 |
| IL/IS/PRE3 | 8.61 ± 0.82 | 16.28 ± 1.75 | 26.85 ± 2.79 | 45.32 ± 2.29 | 57.38 ± 2.46 | 68.69 ± 1.43 |
| IL/IS/PRE4* | 99.16 ± 3.88 | - | - | - | - | - |
In vitro cumulative percent drug release from pre-optimization formulation batches of in situ gel forming depot injections of iloperidone. Mean ± SD, n=3. *1 h- 25.53 %; 3 h- 53.61 %; 6 h- 81.83 %; 12 h- 94.54 % and 24 h- 99.16 %
Table 3: In vitro drug release from pre-optimization formulation batches of iloperidone
SAIB is soluble in some water miscible organic solvents, like ethanol, NMP, DMSO, and triacetin. Out of these solvents, the DMSO and ethanol belong to class 3 category of solvents, which is regarded as safe and less toxic solvent category with lower risk to human health. The NMP, though, is a good solvent for SAIB but it belongs to class 2 solvent category, the use of which is restricted in pharmaceutical products due to its inherent toxicity [24]. Due to these reasons, NMP was not included in the present studies. The relevant particulars of all the above four solvents with regards to their safety considerations are presented in Table 4.
| Solvent | Solvent class | Regulatory status (FDA IIG listing for) |
LD50 (rat) | LD50 (mouse) | ||||
|---|---|---|---|---|---|---|---|---|
| IV | IP | SC | IV | IP | SC | |||
| DMSO | III | IV infusion and SC implants | 5.3 | 8.2 | 12 | 3.8 | 2.5 | NR |
| Triacetin | NR | None (but GRAS listed) | NR | 2.1 | 2.8 | 0.75 | 1.4 | 2.3 |
| Ethanol | III | IV, SC, IM injection | 1.44 | 3.75 | NR | 1.97 | 0.93 | 3.05 |
| NMP | II | SC injection | 0.08 | 2.47 | NR | 0.15 | 3.05 | NR |
FDA is Food and Drug Administration; IIG is inactive ingredients guide, GRAS is generally recognized as safe; NR is not reported; IV is intravenous; IP is intraperitoneal; SC is subcutaneous and IM is intramuscular
Table 4: Toxicological aspects of DMSO, triacetin, ethanol, and NMP[24-26]
Though triacetin is GRAS listed, but it is not included in IIG list for any parenteral product [25]. Considering the LD50 values also it stands inferior to DMSO and ethanol. On comparing the LD50 values, DMSO was found to be a much safer solvent than even ethanol [26]. The in vitro drug release profile of the respective solventbased product as well as their safety considerations as detailed above favored the selection of DMSO as the most functionally effective and safest solvent option among all the four solvents. The composition of SAIB and DMSO was optimized by D-optimal mixture design using Design Expert software. The independent variables along with their levels selected in the designing of depot injection are listed in Table 5.
| Independent variable | Lower level | Upper level |
|---|---|---|
| % Concentration of SAIB (X1) | 50 | 90 |
| % Concentration of DMSO (X2) | 10 | 50 |
Table 5: Independent variables selected for optimization of in situ gel forming depot injection of iloperidone
The 50 % SAIB concentration was taken as the lowest level since at concentrations lower than 50 % SAIB was not found to sufficiently retard the drug release from the depot injection formulation. The upper level of SAIB concentration was kept as 90 % as the formulations with more than 90 % SAIB concentration resulted in an excessively viscous fluid, with poor syringeability. The optimization software suggested 5 formulation trial runs with different concentrations of SAIB and DMSO. The DOE plan of the formulation optimization study is shown in Table 6.
| Formulation batch code |
Independent variable | |
|---|---|---|
| X1 (SAIB %) | X2 (DMSO %) | |
| IL/IS/OP1 | 50 | 50 |
| IL/IS/OP2 | 60 | 40 |
| IL/IS/OP3 | 70 | 30 |
| IL/IS/OP4 | 80 | 20 |
| IL/IS/OP5 | 90 | 10 |
Table 6: Design of experiment plan for formulation optimization of in situ gel forming depot injection of iloperidone
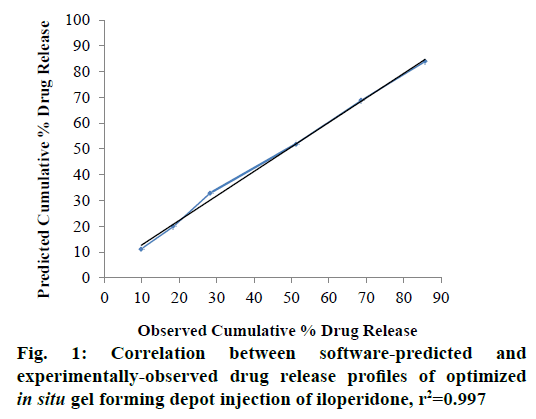
The formulations were prepared and subjected to in vitro drug release study for 30 d. The cumulative percent drug release at the end of day 1 (Y1), day 3 (Y2), day 7 (Y3), day 15 (Y4), day 22 (Y5), and day 30 (Y6) were selected as the response variables. Drug release data obtained are presented in Table 7. The drug release profiles showed that the rate of drug release was consistently reduced, as the amount of SAIB was increased in the formulation. The obtained response variables were entered into the Design Expert software and the numerical optimization of the drug release from the formulations was done with the help of desirability function. For the optimum drug release from the designed in situ gel forming depot injection, the desirability criteria were set as the minimum cumulative percent drug release in 1, 3 and 7 d, and maximum cumulative percent drug release in 30 d. Accordingly, the software provided following polynomial equations to establish the relationship between independent and response variables, Y1 = 0.01248X1+0.55988X2; Y2 = 0.05880X1+0.82900X2; Y3 = 0.16099X1+1.08169X2; Y4 = 0.28876X1+1.04037X2+6.30714×10-3X1X2; Y5 = 0.52802X1+1.40982X2, and Y6 = 0.55560X1 +0.34104X2+0.02162X1X2. The p-values obtained for response variables Y1 to Y6 were 0.0010, 0.0018, 0.0012, 0.0003, 0.0007 and 0.0108, respectively all of which were less than 0.0500. This confirmed that the experimental design model was significant. Further, the software predicted an optimal formulation composition of the in situ gel forming depot injection and its drug release profile with a desirability value of 0.7 as shown in Table 8. The software-predicted optimal formulation was prepared in the laboratory and subjected to in vitro drug release study for 30 d. The results obtained are shown in Table 9. The optimized formulation exhibited a consistent drug release profile with a negligible burst release and 85.71 % cumulative percent drug release in 30 d. The r2 values for predicted and observed drug release profiles were 0.989 and 0.995, respectively. To establish correlation between the experimentallyobserved and software-predicted drug release profiles, a graph was plotted between the predicted and observed drug release data as shown in Figure 1 [27]. The correlation coefficient of 0.997 confirmed a good agreement between the predicted and observed drug release data. To understand the drug release kinetics of the developed optimized in situ gel forming depot injection, the in vitro drug release data were fitted into zero order, first order, Higuchi, and Hixson-Crowell drug release models and the correlation coefficient (r2) with respect to each model was determined to evaluate the accuracy of fit. The best fit of the experimental data was observed in zero order drug release model (r2=0.985), which indicated a near zero order drug release from the developed formulation. The r2 value of Higuchi model was 0.976, which implied that the drug release from the developed in situ gel forming depot system was mainly diffusion controlled [28]. This is substantiated by the fact that SAIB was reported not to undergo any degradation in vitro [16].
| Formulation batch code | In vitro cumulative % drug release | |||||
|---|---|---|---|---|---|---|
| Day 1 (Y1) |
Day 3 (Y2) |
Day 7 (Y3) |
Day 15 (Y4) |
Day 22 (Y5) |
Day 30 (Y6) |
|
| IL/IS/OP1 | 28.63 ± 1.81 | 42.68 ± 2.75 | 60.73 ± 3.79 | 82.06 ± 1.29 | 96.33 ± 2.98 | 99.15 ± 4.42 |
| IL/IS/OP2 | 23.57 ± 3.21 | 38.97 ± 2.23 | 54.10 ± 2.89 | 74.52 ± 3.80 | 87.12 ± 4.80 | 98.76 ± 4.10 |
| IL/IS/OP3 | 17.98 ± 2.30 | 30.19 ± 3.30 | 46.13 ± 3.34 | 64.33 ± 2.56 | 80.94 ± 1.64 | 93.27 ± 5.02 |
| IL/IS/OP4 | 10.25 ± 3.19 | 18.87 ± 2.60 | 31.79 ± 4.22 | 54.01 ± 3.82 | 72.18 ± 2.13 | 87.65 ± 3.08 |
| IL/IS/OP5 | 7.92 ± 1.92 | 14.22 ± 3.75 | 25.85 ± 1.85 | 42.12 ± 4.88 | 59.71 ± 3.46 | 72.19 ± 3.91 |
In vitro cumulative percent drug release from optimized formulation batches of in situ gel forming depot injections of iloperidone. Mean ± SD,
Table 7: In vitro drug release from optimized formulation batches of iloperidone
| Ingredient | Amount |
|---|---|
| Iloperidone | 30 mg |
| SAIB | 81.718 % |
| DMSO | 18.282 % |
Table 8: Composition of software-predicted optimized formulation of in situ gel forming depot injection of iloperidone
| Time | In vitro cumulative % drug release | |
|---|---|---|
| Software predicted | Experimentally observeda | |
| Day 1 | 11.26 | 9.86 ± 0.95 |
| Day 3 | 19.96 | 18.47 ± 1.43 |
| Day 7 | 32.93 | 28.29 ± 3.48 |
| Day 15 | 52.04 | 51.28 ± 3.22 |
| Day 22 | 68.92 | 68.72 ± 3.48 |
| Day 30 | 83.94 | 85.71 ± 4.19 |
Software-predicted and experimentally-observed in vitro cumulative percent drug release of the optimized in situ gel forming depot injection of iloperidone. aMean ± SD, n=3
Table 9: Software-predicted and experimentally-observed drug release of iloperidone
In summary, the SABER® technology was explored in this study to develop an in situ gel forming one month depot injection formulation of iloperidone using D-optimal mixture design technique. The Design Expert software predicted the optimum formulation composition comprising of 81.718 % SAIB and 18.282 % DMSO. The optimized injection formulation experimentally yielded a zero order drug release profile with 85.71 % cumulative percent drug release in 30 d. The experimentally-observed and software-predicted drug release profiles were in good agreement with a correlation coefficient of 0.997.
Acknowledgements
Authors would like to acknowledge M/S Sun Pharmaceutical Industries Ltd., Mumbai, India for supplying iloperidone and M/S Eastman Chemical Company, Kingsport, USA for supplying sucrose acetate isobutyrate as gift samples.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Weiden PJ. Iloperidone for the treatment of schizophrenia: An updated clinical review. Clin Schizophr Relat Psychoses 2012;6(1):34-44.
- Cutler AJ, Kalali AH, Weiden PJ, Hamilton J, Wolfgang CD. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol 2008;28(2 Suppl 1):S20-8.
- Citrome L. Iloperidone: Chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability, regulatory affairs, and an opinion. Expert Opin Drug Metab Toxicol 2010;6(12):1551-64.
- Wieckhusen D, Glausch A, Ahlheim M. Injectable depot formulation comprising crystals of iloperidone. United States Patent; US 8614232 B2. 2013.
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: Epidemiology, contributing factors and management strategies. World Psychiatry 2013;12(3):216-26.
- Valenstein M, Ganoczy D, McCarthy JF, Myra Kim H, Lee TA, Blow FC. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: A retrospective review. J Clin Psychiatry 2006;67(10):1542-50.
- Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence 2013;7:1171-80.
- Reynolds RC, Chappel CI. Sucrose acetate isobutyrate (SAIB): historical aspects of its use in beverages and a review of toxicity studies prior to 1988. Food Chem Toxicol 1998;36:81-93.
- Reynolds RC. Metabolism and pharmacokinetics of sucrose acetate isobutyrate (SAIB) and sucrose octaisobutyrate (SOIB) in rats, dogs, monkeys or humans: a review. Food Chem Toxicol 1998;36(2):95-9.
- Okumu FW, Dao le N, Fielder PJ, Dybdal N, Brooks D, Sane S, et al. Sustained delivery of human growth hormone from a novel gel system: SABER. Biomaterials 2002;23(22):4353-8.
- Lu Y, Yu Y, Tang X. Sucrose acetate isobutyrate as an in situ forming system for sustained risperidone release. J Pharm Sci 2007;96:3252-62.
- Hadj A, Hadj A, Hadj A, Rosenfeldt F, Nicholson D, Moodie J, et al. Safety and efficacy of extended-release bupivacaine local anaesthetic in open hernia repair: a randomized controlled trial. ANZ J Surg 2012;82:251-7.
- Kempe S, Mader K. In situ forming implants - an attractive formulation principle for parenteral depot formulations. J Control Release 2012;161:668-79.
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res 2004;21:201-30.
- Arthur JT. Sucrose acetate isobutyrate (SAIB) for parenteral delivery. In: Rathbone MJ, Hadgraft J, Roberts MS, editors. Modified-release drug delivery technology. Drugs and the Pharmaceutical Sciences. 2nd ed. New York: Marcel Dekker Inc.; 2002. p. 679-87.
- Okumu F. Sustained release formulations. United States Patent; US 6992065 B2. 2006.
- Cook J, Cruanes MT, Gupta M, Riley S, Crison J. Quality-by-design: Are we there yet? AAPS PharmSciTech 2014;15(1):140-8.
- Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J 2014;16:771-83.
- Singh B, Kumar R, Ahuja N. Optimizing drug delivery systems using systematic "Design of experiments." Part I: Fundamental aspects. Crit Rev Ther Drug Carrier Syst 2005;22(1):27-105.
- Zen NI, Abd Gani SS, Shamsudin R, Masoumi HR. The Use of D-optimal Mixture Design in Optimizing Development of Okara Tablet Formulation as a Dietary Supplement. Sci World J 2015;2015:684319.
- Wang L, Wang A, Zhao X, Liu X, Wang D, Sun F, et al. Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism. Int J Pharm 2012;427:284-92.
- Geng Z, Luo X, Zhang Z, Li H, Tian J, Yu Z. Study of an injectable in situ forming gel for sustained-release of Ivermectin in vitro and in vivo. Int J Biol Macromol 2016;85:271-6.
- Conti B, Genta I, Giunchedi P, Modena T. Testing of “in vitro” dissolution behaviour of microparticulate drug delivery systems. Drug Dev Ind Pharm 1995;21:1223-33.
- USFDA. International Conference on Harmonisation; Final recommendations on the revision of the permitted daily exposures for two solvents, N-methylpyrrolidone and tetrahydrofuran, according to the maintenance procedures for the guidance Q3C Impurities: Residual Solvents; Availability. Notice. Fed Regist 2003;68:64352-3.
- USFDA. Inactive ingredient guide. U.S. Food and Drug Administration. 2017. [Last accessed on 2017 Mar 06]. Available from: http://www.accessdata.fda.gov/scripts/cder/iig.
- Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of pharmaceutical excipients. 5th ed. London, Chicago: Pharmaceutical Press, American Pharmacists Association; 2009.
- Nahata T, Saini TR. D-optimal designing and optimization of long acting microsphere-based injectable formulation of aripiprazole. Drug Dev Ind Pharm 2008;34:668-75.
- Higuchi WI. Analysis of data on the medicament release from ointments. J Pharm Sci 1962;51:802-4.