- *Corresponding Author:
- S. Janakidevi
A. U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India
E-mail: janaki.sirisolla@gmail.com
| Date of Submission | 02 December 2016 |
| Date of Revision | 15 October 2017 |
| Date of Acceptance | 24 May 2018 |
| Indian J Pharm Sci 2018;80(4):604-609 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study is aimed to develop and characterize microsponge-based novel colon-specific drug delivery systems containing 5-amino salicylic acid for the treatment of inflammatory bowel disease. Initially, microsponges of 5-amino salicylic acid were prepared by quasi-emulsion solvent diffusion method using Eudragit RS100, Eudragit S-100 and Eudragit L100. Different formulations of microsponges using three acid resistant polymers, Eudragit S100, Eudragit L100, Eudragit RS100 in drug:polymer ratios of 1:1, 1:0.5, 1:0.25 with each polymer were prepared and evaluated for physicochemical, morphological characteristics and in vitro parameters. Among these nine formulations, ES1, EL1 and ERS1 were selected, sieved and compressed into tablets, T-ES1, T-EL1, T-ERS1 and evaluated. Among the 3 batches of tablet formulations prepared, T-ES1 showed the best release rate for the drug, which followed zero order release kinetics with diffusion case II transport mechanism.
Keywords
Inflammatory bowel diseases, microsponges, colon
Inflammatory bowel diseases (IBD) include two major forms of chronic intestinal disorders namely Crohn’s disease (CD) and ulcerative colitis (UC). CD mostly affects the ileum and colon while UC involves the rectum and may affect a part or the entire colon [1-3]. Colon targeting is essential to provide more effective therapy to colon diseases, such as irritable bowel syndrome, colon cancer and IBD [4-6]. A colon-specific drug delivery system should prevent drug release in the stomach and small intestine and initiate abrupt onset of drug release upon entry in to the colon [7,8].
The microsponge drug delivery system has many favourable characteristics such as enhanced stability due to high degree of crosslinking, reduced side effects due to targeted and modified drug release, and also protects the entrapped active ingredients from physical and environmental degradation, which makes it a suitable drug delivery carrier. Microsponges are also capable of delivering pharmaceutical active ingredients efficiently at a minimal dose to targeted site, which reduces severe systemic degradation [9,10]. Mesalamine (5-aminosalicylic acid, mesalazine) is an antiinflammatory drug used to treat IBD [11], which is readily metabolized in the intestinal mucosal wall and in the liver and this metabolism can be overcome by formulating mesalamine as colontargeted microsponges prepared using acid resistant polymers [12].
Materials and Methods
Mesalamine was received as a gift sample from Agro Chemicals Ltd., Hyderabad, India, while Eudragit S100, Eudragit L100, Eudragit RS100 were gift samples from Hetero Labs, Hyderabad, India. Polyvinyl alcohol and ethanol were procured from Loba Chemie, and Qualigens Fine Chemicals, Mumbai, India, respectively. All other ingredients used were of analytical grade, and were used as procured.
Fourier-transform infrared (FTIR) spectroscopy
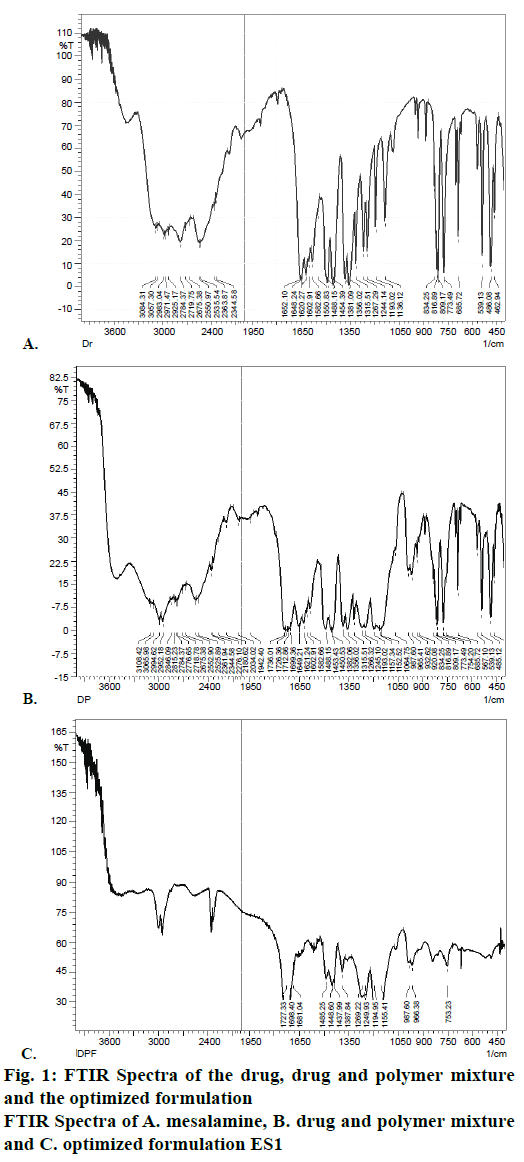
The FTIR spectra of samples were obtained using a FTIR formulations spectrophotometer (Perkin Elmer). Pure drug, individual polymers and optimized formulations were subjected to FTIR analysis. About 2-3 mg of sample was mixed with dried potassium bromide of equal weight and compressed to form a KBr disk. The samples were scanned from 500 to 4000 cm-1. FTIR spectra of the drug, physical mixture of drug and Eudragit S100, formulation ES1 were recorded in potassium bromide disc using a Shimadzu Model 8400 FTIR spectrometer to ascertain compatibility.
Drug-loaded microsponge preparation
Microsponges were prepared by quasi-emulsion solvent diffusion method. It consisted of two phases, the internal and the external phase. Polymer and plasticizer was dissolved in a suitable organic solvent to form the internal phase. The drug was added to the internal phase with gradual stirring (1000 rpm). The internal phase was then poured into the external phase containing polyvinyl alcohol (30 000-70 000) solution in water. After 8 h of stirring, the formed microsponges were filtered and dried at 40° for 12 h. Various formulation batches were prepared as shown in Table 1 [13].
| Formulation code | Drug (mg) | Eudragit S100 (mg) | Eudragit L100 (mg) |
Eudragit RS 100 (mg) |
Ethanol (ml) | PVA (mg/ml) |
Total (mg) |
|---|---|---|---|---|---|---|---|
| ES-1 | 400 | 400 | ---- | -- | 5 | 0.005 | 800 |
| ES-2 | 400 | 200 | --- | -- | 5 | 0.005 | 600 |
| ES-3 | 400 | 100 | -- | -- | 5 | 0.005 | 500 |
| EL-1 | 400 | -- | 400 | -- | 5 | 0.005 | 800 |
| EL-2 | 400 | -- | 200 | -- | 5 | 0.005 | 600 |
| EL-3 | 400 | -- | 100 | -- | 5 | 0.005 | 500 |
| ERS-1 | 400 | -- | ---- | 400 | 5 | 0.005 | 800 |
| ERS-2 | 400 | -- | --- | 200 | 5 | 0.005 | 600 |
| ERS-3 | 400 | -- | -- | 100 | 5 | 0.005 | 500 |
Table 1: Composition of microsponge formulations
Evaluation of microsponges
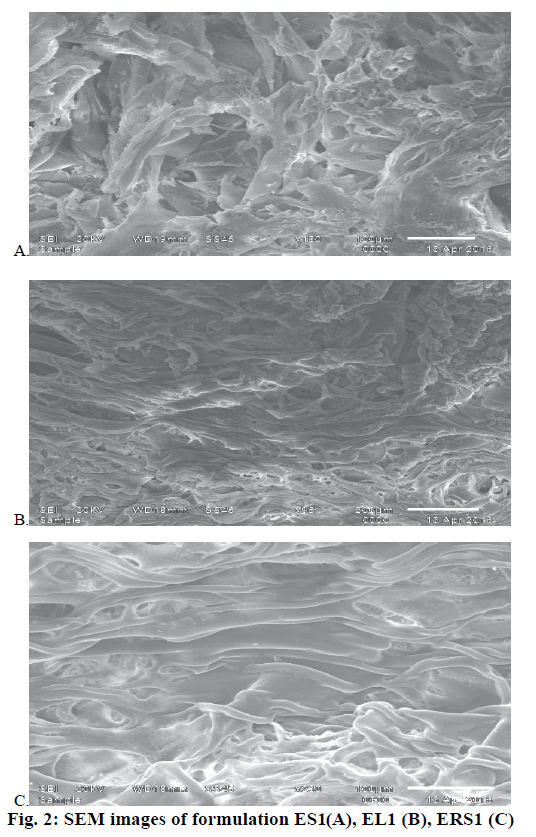
The morphology and surface characteristics of the microsponges were studied using scanning electron microscopy (SEM). All the samples were coated with gold palladium alloy under vacuum. Coated samples were then examined using LEO 430 SEM analyser [13-15]. For the determination of actual drug content, the weighed amount of drug-loaded microsponges (100 mg) was kept in 100 ml phosphate buffer pH 6.8 for 12 h with continuous stirring. The samples were filtered using Whatman filter and the samples were analysed at 300 nm against blank using a UV-spectrophotometer (UV 1700, Shimadzu) [16,17].
For the determination of encapsulation efficiency (EE), percent yield (PY) and drug loading (DL), a weighed amount of drug-loaded microsponges (100 mg) was placed in 100 ml phosphate buffer pH 6.8 for 12 h with continuous stirring. The samples were filtered and analysed at 300 nm against blank on a UV spectrophotometer [9]. The EE, PY, and DL were calculated using the following Eqns., EE = mass of drug in microsponge/initial mass of drug×100; PY = mass of obtained microsponges/initial mass of drug+initial mass of polymer×100; DL = mass of drug in microsponges/mass of microsponges×100.
In vitro release studies were carried out in a USP basket apparatus with stirring rate 50 rpm at 37±0.5°. Initial drug release was carried out in 900 ml of 0.1 N HCl for 2 h followed by in phosphate buffer pH 6.8 for the next 8 h. Samples were withdrawn at regular intervals of time and each time were compensated by adding equal volume of fresh dissolution medium to maintain the sink condition. The samples were analysed spectrophotometrically at a wavelength of 300 nm. Dissolution tests were performed in triplicate for each sample [9].
Preparation of colon-specific tablet formulations
The optimized microsponges were further compressed into core tablets consisting of drug-loaded microsponges containing 300 mg drug and other excipients like lactose and magnesium stearate using the direct compression technique. All tablet constituents were weighed and mixed in a mortar for 15 min. The final powder mix is compressed using round flat punches on a tablet punching machine by applying required compression pressure. Core tablet formulations are given in Table 2 [18].
| Core tablet formulation codes | Microsponge formulations (mg) | Lactose (mg) | Magnesium stearate (mg) | ||
|---|---|---|---|---|---|
| T-ES1 | 350 | -- | -- | 142 | 8 |
| T-EL1 | -- | 390 | -- | 102 | 8 |
| T-ERS1 | -- | -- | 440 | 52 | 8 |
Table 2: Composition of microsponges compressed core tablets
Evaluation parameters of microsponge-loaded tablets
Three tablets were picked from each formulation randomly and thickness was measured individually. It is expressed in millimeter and standard deviation (SD) was also calculated. The hardness of the tablets was determined using a Pfizer hardness tester. It is expressed in kg/cm2. Three tablets were randomly picked and hardness of the same tablets from each formulation was determined. The mean and SD values were also calculated [19]. Friability of tablets was determined using a Roche Friabilator. Ten tablets were weighed (Winitial) and transferred into the friabilator. The friabilator was operated at 25 rpm for 4 min or for 100 revolutions [19]. The tablets were weighed again (Wfinal). The % friability was then calculated using the Eqn., F = Winitial–Wfinal/Winitial×100.
For the weight variation test, 20 tablets were selected randomly from each formulation and weighed individually to check for weight variation [19]. The following percent deviation in weight variation is allowed: % deviation = average weight–weight of tablet/average weight×100.
In vitro dissolution studies were carried out using the USP XXIII tablet dissolution test apparatus. In vitro drug release studies of colon-specific tablet formulations were carried out using USP basket apparatus with stirring rate 50 rpm at 37±0.5°. For the first 1 h, simulated gastric fluid of pH 1.2 was used, followed by a mixture of simulated gastric and intestinal fluid (pH 4.5) for the next 2 h, after which simulated intestinal fluid of pH 6.8 for 2 h followed by simulated intestinal fluid of pH 7.5 for 1 h was used. Pectinex Ultra SP-L was added to the dissolution medium at 6th h in order to simulate the enzymatic action of the colonic bacteria. Samples were withdrawn periodically and compensated with an equal amount of fresh dissolution media. The samples were analysed for the drug content by measuring absorbance at 300 nm using a UV spectrophotometer [19].
Results and Discussion
Microsponges prepared using three different polymers were evaluated using various tests and the results obtained were reported here. Prepared microsponges were characterized for physical appearance, microscopic evaluation, EE, PY, DL, and in vitro drug release.
FTIR spectra were recorded to assess the compatibility of the drug and excipients. FTIR spectra of the drug, physical mixture of drug and Eudragit S100 and optimized formulations ES1 were given in Figure 1. It is clear from the FTIR that the characteristic peaks of the drug were also present in the formulation depicting no incompatibility between the drug and polymers in the formulation. The microsponge formulations ES1, EL1, ERS1 were visualized by SEM to assess the morphology of microsponges. SEM image revealed spherical and porous surface as shown in Figure 2.
Extent of DL, PY, and EE of various batches of microsponges were evaluated and the results were presneted in Table 3. PY ranged between 69±0.04 to 84±0.23 % for formulation with Eudragit S100, 58±0.53 to 65±0.26 % for formulations with Eudragit L100 and 62±0.29 to 72±0.034 % for formulations with Eudragit RS100. Different formulations showed EE values that varied between 90±0.16 and 95±0.23 %. DL values for different formulations were in the range of 80±0.023 to 53±0.023 %.
| Formulation code | Production yield (% ±SD) |
Theoretical drug content (%) |
Actual drug content (% ±SD) |
Encapsulation efficiency (% ±SD) |
|---|---|---|---|---|
| ES-1 | 84±0.23 | 84 | 80±0.023 | 95±0.23 |
| ES-2 | 78±0.45 | 78 | 74±0.03 | 94±0.87 |
| ES-3 | 69±0.04 | 69 | 65±0.01 | 94±0.20 |
| EL-1 | 65±0.26 | 65 | 60±0.05 | 92±0.30 |
| EL-2 | 61±0.13 | 61 | 55±0.09 | 90±0.16 |
| EL-3 | 58±0.53 | 58 | 53±0.023 | 91±0.37 |
| ERS-1 | 72±0.034 | 72 | 68±0.005 | 94±0.44 |
| ERS-2 | 68±0.33 | 68 | 62±0.003 | 91±0.17 |
| ERS-3 | 62±0.29 | 62 | 56±0.014 | 90±0.32 |
Table 3: Evaluation parameters of microsponges
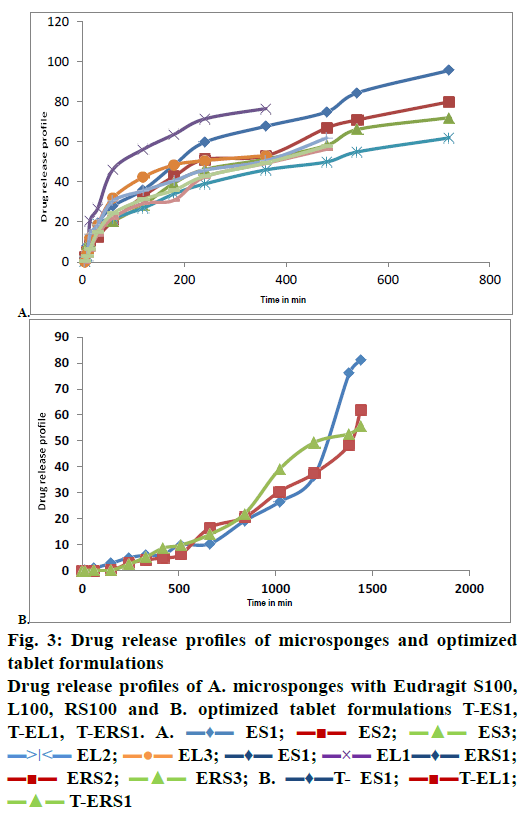
The results of dissolution studies indicated that formulation ES-1 released 95.7 % of drug in 12 h, ES-2 released 80 %, and ES-3 released 72.1 %, while the formulation EL-1 released 76.62 % in 6 h, EL-2 released 62.025 % in 12 h and EL-3 released 53.152 % in 6 h as shown in Figure 3A. From the data obtained, it could be concluded that the formulation ES-1, which released 95.7 % of drug in 12 h was the best formulation. Comparing the dissolution profiles of nine formulations, one was selected from each batch i.e. ES1 from batch one, EL1 from batch two, ERS1 from batch 3. On comparing all the three selected formulations, it was found that ES1 released 95.7± 0.12 % of the drug at the end of 12 h period. The optimized microsponges were directly compressed in to tablets and the hardness, weight variation, thickness, friability and in vitro dissolution of these tablets were evaluated.
Hardness of all the formulations was found to be in the range of 3.1-3.5 kg/cm2. Weight of the tablets was found to be in the range as per limits of Indian Pharmacopoeia. Thickness of the matrix tablets was measured with a Vernier callipers (Table 4). The drug content in the prepared sustained release matrix tablets was found to be in the range of 97.32-99.87 %. The results are shown in Table 4. The percent friability of the prepared mesalamine-loaded microsponge tablets was calculated and the results exhibited less than one percent deviation (Table 4). All the tablets passed the friability test.
| Formulation code | Thickness (mm, n=3) | Weight variation (mg, n=3) |
Hardness (kg/cm2, n=3) | Friability (%, n=10) | Drug content (%, n=10) |
|---|---|---|---|---|---|
| T-ES1 | 2.89±0.32 | 496±3.39 | 3.2±0.41 | 0.25±0.02 | 90.15±0.44 |
| T-EL1 | 2.93±0.35 | 495±2.54 | 3.2±0.45 | 0.38±0.04 | 62.05.52±0.41 |
| T-ERS 1 | 2.95±0.28 | 497±2.65 | 3.1±0.57 | 0.36±0.03 | 62.69±0.43 |
Table 4: Characteristics of microsponge-loaded tablets
The results of dissolution studies indicated that formulations T-ES1 releases 81.27 % of drug at the 24th h, T-EL1 releases 62.05 % and T-ERS1 released 55.59 % of drug in 24 h. Figure 3B showed the drug release profiles of all formulations and tablets prepared. From the data obtained, it could be concluded that the formulation T-ES1, which released 81.27 % drug in 24 h was the best formulation.
In vitro drug release and release mechanism were studied for these three formulations. Release kinetics followed zero order kinetics and the release mechanism was observed to be the diffusion mechanism. The type of diffusion was super case-II transport, hence the prepared microsponge tablets, T-ES1, T-EL1, and T-ERS1 were found to extend drug release as expected (Table 5). When these three formulations were compared with each other, T-ESI was found to release the drug to the maximum extent (82.17 %) at the end of 24 h.
| Tablet | Zero order | First order | Higuchi | Hixson-Crowell | Korsmeyer-Peppas | |||
|---|---|---|---|---|---|---|---|---|
| R | K0 (%/H) | R | K1 (HR-1) |
R | R | R | N | |
| T-ES1 | 0.977 | 0.048 | 0.832 | 0.0009 | 0.802 | 0.938 | 0.984 | 1.79 |
| T-ELI | 0.964 | 0.038 | 0.930 | 0.0009 | 0.874 | 0.968 | 0.968 | 1.73 |
| T-ERS1 | 0.905 | 0.041 | 0.965 | 0.0009 | 0.897 | 0.856 | 0.959 | 1.71 |
Table 5: Mechanism of drug release studies of microsponge-loaded tablets
The present work aimed to develop colon-targeted mesalamine-loaded microsponges to serve the purpose of increasing the drug release by using ethanol or Eudragit as the internal phase and 5 % w/v PVA as the external phase. Nine microsponges were prepared using three different polymers, among these; three formulations, ES1, EL1 and ERS1 were selected and their dissolution profiles were compared. Among those three formulations, ES1 gave acceptable results. These three selected formulations were sieved and compressed in to tablets, T-ES1, T-EL1, T-ERS1 and the compressed tablets were evaluated. Among the 3 batches of tablets formulations, T-ES1 gave best release rate of mesalamine, with highest percent of the drug released at the end of 24 h compared to the other 2 tablets. It was found that the drug release followed zero order release kinetics and diffusion with case-II transport mechanism. These results indicated that microsponges could be used as efficient means of formulation to enhance drug delivery and bioavailability of a drug in the colon and this approach could produce efficient carriers for colon targeting.
Financial support and sponsorship
The author thank full to DST-WOSA for providing financial assistance.
Conflicts of interest
There are no conflicts of interest.
References
- Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett 2014;161:231-5.
- Prasad YG, Lei WX, Zhanju L. Psychological Stress Exacerbates Development of Inflammatory Bowel. Biomed Lett 2016;2(1):53-9.
- Goyal N, Rana A, Ahlawat A, Bijjem KR, Kumar P. Animal models of inflammatory bowel disease: a review. Inflammopharmacology 2014;22:219-33.
- Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol 2012;3:107.
- Redondo-Sendino A. An uncommon case of proximal Crohn's disease. Semergen 2012;38:539-42.
- Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am 2002;31:1-20.
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119-24.
- Asghar LF, Chandran S. Multiparticulate formulations approach to colon specific drug delivery: current perspectives. J Pharm Pharm Sci 2006;9:327-38.
- Comoğlu T, Gönül N, Baykara T. Preparation and in vitro evaluation of modified release ketoprofen microsponges. Farmaco 2003;58:101-6.
- Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. The micro sponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm 2006;308:124-32.
- Kruis W, Bar-Meir S, Feher J, Mickisch O, Mlitz H, Faszczyk M. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol 2003;31;1:36-4.
- Sinha VR, Kumria R. Coating polymers for colon specific drug delivery: a comparative in vitro evaluation. Acta Pharm 2003;53:41-7.
- Sareen R, Nath K, Jain N, Dhar KL. Curcumin loaded microsponges for colon targeting in inflammatory bowel disease: Fabrication, optimization, and in vitro and pharmacodynamic evaluation. Biomed Res Int 2014;340701.
- Jain V, Singh R. Design and characterization of colon-specific drug delivery system containing paracetamol microsponges. Arch Pharm Res 2011;34:733-40.
- Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. The microsponge delivery system of benzoyl peroxide: Preparation, characterization and release studies. Int J Pharm 2006;308:124-32.
- Devrim B, Canefe K. Preparation and evaluation of modified release ibuprofen microspheres with acrylic polymers (Eudragit) by quasi emulsion Solvent diffusion method: effect of variables. Acta Pol Pharm 2006;63:521-34.
- Nokhodchi A, Jelvehgari M, Siahi MR, Mozafari MR. Factors affecting the morphology of benzoyl peroxide microsponges. Micron 2007;38:834-40.
- Orlu M, Cevher E, Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm 2006;318:103-17.
- Cooper J, Gunn C. Powder flow and compaction. In: Carter SJ, editor. Tutorial pharmacy, New Delhi: CBS Publishers and Distributors; 1986. p. 211-33.
 ES1;
ES1;  ES2;
ES2;  ES3;
ES3;  EL2;
EL2; EL3;
EL3; ES1;
ES1; EL1
EL1  ERS1;
ERS1; 



