- *Corresponding Author:
- K. Sathishkumar
Department of Chemical Engineering, Sri Sivasubramaniya Nadar College of Engineering, Kalavakkam, Kancheepuram-603 110, India
E-mail: sathishkumark@ssn.edu.in
| Date of Submission | 13 August 2015 |
| Date of Revision | 10 January 2016 |
| Date of Acceptance | 24 February 2016 |
| Indian J Pharm Sci 2016;78(1):136‑142 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The present research was aimed to develop and characterize a sustained release dry powder inhalable formulation of salbutamol sulphate. The salbutamol sulphate microparticles were prepared by solvent evaporation method using biodegradable polymer poly (D,L-lactic-co-glycolic acid) to produce salbutamol sulphate microparticle mixed with carrier respirable grade lactose for oral inhalation of dry powder. The drug content were estimated to produce 1 mg sustained release salbutamol sulphate per dose. Total four formulations K1, K2, K3 and K4 were prepared with 1:1, 1:2, 1:3, 1:4 ratio of salbutamol sulphate:poly (D,L-lactic-co-glycolic acid). The developed formulations were studied for physicochemical properties, in vitro drug relase and Anderson cascade impaction studies. The prepared formulations effectively releases drug for 12 h in diffusion bag studies. Based on dissolution performance the 1:1 ratio of salbutamol sulphate:poly (D,L-lactic-co-glycolic acid) produces in vitrorelease 92.57% at 12 h and having particle size of microparticles (D0.5μm) 5.02±0.6 and the pulmonary deposition of dry powder 34.5±3.21 (respiratory fraction in percentage).
The inhalation therapy records were found in ancient Indian culture. In olden age these therapy contains aromatic fumes and vapours created from plant or from burning organic materials. The first successful dry powder inhaler (DPI) was developed almost 30 years ago. May be the Hippocrates was the first person to describe the inhaler device. The first records described the aerosol adrenaline was administered using squeeze bulb nebulizer. Asthma caused by an allergic reaction because of allergen such as animal hairs, plant pollens, dust, feathers and certain chemicals and foods [1]. Asthma was a chronic inflammatory disorder of the airways in which many cells play a role, in particular mast cells, eosinophils and lymphocytes [2]. In susceptible individuals, this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness and cough particularly at night and/or in the early morning at atmospheric air pressure. The inflammation also causes an associated increase in airway responsiveness to a variety of stimuli.
Asthma classified as intrinsic (chronic) and extrinsic (allergic) [3]. During an asthma, spasmodic contraction of bronchial muscle constricts the airway and thick sticky mucus secretion reduces the airway. Asthma controlled by a combination of therapies. Oxygen is essential for human survival, 21% of oxygen must be in inspired air. In asthma patients oxygen was decreased due to lack of ventilation which cause alveolar hypoventilation (hypoxeima [4]), i.e., failure to maintain the partial pressure of oxygen in the alveoli at the normal level condition which give rise to rapid and shallow breathing. At this condition life will be saved by administration of therapeutic gas oxygen with bronchodilator. Drug taken regularly by inhaler or by mouth will help to prevent attacks. In extreme cases mechanical respirator required. Total amounts of inhaled powders (including active drug and any excipients) are usually less than 10-20 mg [5].
Salbutamol has a potent bronchodilator effect by the stimulation of β2 receptors. It is administered by mouth, inhalation and injection. The normal dose for inhalation of salbutamol sulphate was 100 μg and 2 to 4 mg by mouth, 0.6 mg every 4 h by intramuscular injection. The pulmonary route by oral inhaler delivery of salbutamol sulphate was considered as another route of non invasive systemic drug delivery method. This route was also used as alternate to oral delivery. Salbutamol sulphate is hydrophilic drug [6]. It is a short acting beta 2 adrenoreceptor stimulant with plasma half life of 4-6 h, which requires frequent dosing for daily management of asthma. The aerosol route of drug delivery was a twentieth century innovation.
The DPIs were single dose units containing micronized drugs blended with lactose carrier particles in single gelatin capsules to be inserted in to the device by the patient immediately before inhalation use. Rotahaler, spinhaler, cyclohaler/aerolyser were well known single dose powder inhaler. In rotahaler device capsule inserted and broken into two halves by twisting the mouthpiece. The powder falls into the body of the inhaler and patient inhale through the mouth piece to scatter the powder and deliver small quantity of the micronized drug particles into the lung. The powder properties played active role in this inhalable powder formulation for the deposition to the deep lung depends based on particle size, shape, morphology and density. The biocompatible excipients selection was considered as must to produce a desired dissolution and the improved deposition of desirable respirable particles in to the mini airways of the lung. The sustained release inhalation formulation was developed as one of the application of particle engineering, which was not marketed yet. The sustained release drug delivery releases the salbutamol over an extended period of time and thereby improves therapy by the compliance of asthma patients. In this formulation drug applied directly to the pulmonary system. So salbutamol dose was decreased and also side effects of salbutamol was reduced [7-9].
Materials and Methods
Salbutamol sulphate (SS) was obtained as gift sample from Fourrts (India) laboratories Pvt Ltd, Chennai and poly (lactic-co-glycolic) acid (PLGA 50:50, MW:45KD was obtained from Brimingham polymer Inc, USA. Polyvinyl alcohol was obtained from Orchid Pharmaceuticals, Chennai. Dichloromethane, methanol and acetonitrile was obtained from Qualigens, Mumbai and potassium dihydrogen orthophosphate, sodium chloride and sodium hydroxide were obtained from S. D. Fine-Chem Ltd., Mumbai, India.
Preparation of microparticles:
The solvent evaporation method involves preparation of o/w emulsion between organic phase consisting of PLGA (50:50; 45KD) in dichloromethane (DCM), to this mixture salbutamol sulphate solution was added and mixed by sonication using probe sonicator and injected this mixture drop wise into the aqueous phase containing 2% w/v PVA. The organic phase was emulsified in aqueous phase by homogenization at 10 000 rpm for 10 min using a homogenizer (Viritis Cyclone IQ, USA). The emulsion was stirred for 12 h at 25±2° using magnetic stirrer to ensure complete evaporation of dichloromethane. The microparticles thus prepared were recovered by centrifugation (15 000 rpm, 20 min, 4°). The precipitate was washed completely removed polyvinyl alcohol. The product dispersed in cold water and recovered by lyophilisation. The 4 different batches of microparticles were prepared by keeping organic phase to aqueous phase ratio as (OP:AP::1:5) and varying drug:polymer ratios of Table 1.
| Formulation code | SS: PLGA | Weight of salbutamol sulphate (mg) | Weight of PLGA (mg) | Volume. of DCM (ml) - OP | AP Vol. (2% PVA) (ml) -AP | Percentage yield |
|---|---|---|---|---|---|---|
| K1 | 1:1 | 50 | 50 | 5 | 25 | 82.15±1.81 |
| K2 | 1:2 | 50 | 100 | 5 | 25 | 80.23±2.12 |
| K3 | 1:3 | 50 | 150 | 5 | 25 | 79.34±1.97 |
| K4 | 1:4 | 50 | 200 | 5 | 25 | 79.82±2.06 |
Volume ratio of OP: AP=5 ml: 25 ml (1:5), DCM: Dichloromethane, PLGA: poly (lactic-co-glycolic), SS: salbutamol sulphate, PVA: polyvinyl alcohol, OP: organic phase, AP: aqueous phase
Table 1: Formulation of salbutamol poly (lactic co glycolic) microparticles
Dry powder inhaler preparation:
The physical mixture of microparticle with coarse carrier lactose inhalable grade lactohale to enhance aerosol properties for the production of respirable salbutamol sustained release powder for inhalation.
Fourier transform infrared spectroscopy:
Infrared spectroscopy was used to determine various functional groups of the drug molecule. In general the functional groups can exhibit changes as a result of processing drug formulations. The resulting FTIR spectra graphs of salbutamol sulphate, and Trials K1, K2, K3 and K4 were given and discussed in fig. 1a-e.
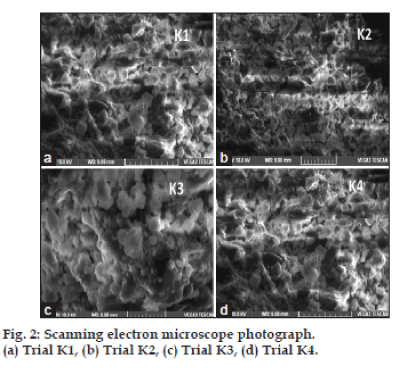
Scanning electron microscopy:
The surface morphology and shape of the prepared microparticles were investigated with scanning electron microscopy (VEGA3 LMU, TESCAN) with a maximum magnification of 10,00,000*X and a resolution 3 nm at a maximum applied voltage of 30 kV. The electronic images were recorded digitally at higher magnification. Scanning electron microscopy was used to visualize the particle diameter, structural and surface morphology of the DPI microparticles. The powders were kept on adhesive coated, 6.25 mm radius aluminium stubs. The remaining powder was removed by tapping the stubs and blowing a jet of particle free compressed gas. Then the specimens were examined in the scanning electron microscope which will operated with maximum vacuum and with accelerating voltage of 5-15 KV with a specimen working distance of 12 min.
Entrapment efficiency:
The drug content was determined from the microparticles. The SS was extracted from microparticles with sodium hydroxide (0.1 M) after dissolving the microparticles in acetonitrile. After suitable dilutions, the SS content was measured in a UV/Vis spectrophotometer (Jasco) at 278 nm. Entrapment efficiency was calculated using the following formula: Entrapment efficiency=(estimated % drug content)/(% drug content (theoretical))×100.
Percent yield:
The percentage yield was calculated by using the following formula. Percentage yield=(Actual wt. of microspheres)/(Wt. of starting materials)×100.
Particle size determination:
The laser diffraction method was used to estimate the particle size of microparticles. The Helos particle size analyser Vibro/Rodos drug dispersion system: Sympatec Gmbh systems were used to measured particle size. Roughly 100 mg of the powder was used to achieve the required obscuration of 5%. The particle size data obtained were represented as D0.5.
Anderson cascade impactor:
Anderson cascade impactor (ACI) deals with particle size as per pharmacopoeial specification. This impactor has eight stages stimulating the diverse parts of the human pulmonary system [10]. By analyzing the amount of drug deposited on the eight stages the fine particle dose and respiratory fraction can be calculated. ACI has induction port, pre separator, seven stages and filter. It operate on the principle of inertial impaction i.e., separation was provided on the basis of differences in inertia – a function of aerodynamic particle size and velocity. The ACI was connected with vacuum pump equipped with a flow meter. The airflow was 60 l/min. The emitted dose (%), respiratory fraction (%), total recovery, mass median aerodynamic diameter were calculated by determining the quantity of salbutamol by UV spectrophotometer of a wavelength of 278 nm. The respirable particle fraction (RF) and emission dose (ED) were calculated to describe the inhalation properties of DPIs. Six replicates of the measurements were performed. The ACI operation conditions were given in Table 2.
| ACI parameters | ACI results |
|---|---|
| Flow rate (L/min) | 60 |
| Time per actuation (s) | 4 |
| Volume per actuation (L) | 4 |
| Cut-off diameter (μm) | |
| Stage 0 | 5.7 |
| Stage 1 | 4.6 |
| Stage 2 | 3.2 |
| Stage 3 | 2.1 |
| Stage 4 | 1.3 |
| Stage 5 | 0.7 |
| Stage 6 | 0.39 |
| Stage 7 | 0.15 |
| Stage 8 | Filter |
| ACI: Anderson cascade impactor |
ACI: Anderson cascade impactor
Table 2: Operating conditions and theoretical cut-off diameters of anderson cascade impactor.
In vitro drug release study:
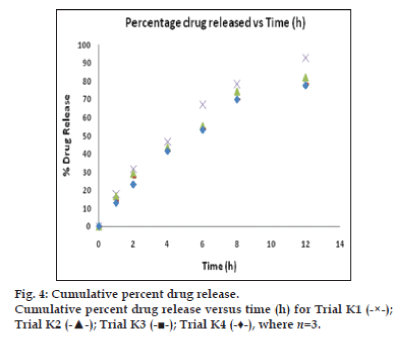
The In vitro dissolution of salbutamol sulphate from the microparticles were analysed by a dialysis bag diffusion method [11]. The In vitro release [12-14] was carried out with phosphate buffer saline (PBS) pH 7.4 as the diffusion medium and a dialysis membrane of 14 kDa molecular weight (Himedia, Mumbai, India) was used. An aqueous dispersion equivalent to 10 mg of salbutamol sulphate microsphere was kept in a dialysis bag and sealed at both ends. The dialysis bag was immersed in 250 ml of diffusion medium and stirred at 100 rpm. Samples were withdrawn at predetermined time intervals, and the receptor phase was replenished with an equal volume of blank after each sample was withdrawn. Samples were filtered through 0.46 μm filter and appropriately diluted with pH 7.4 PBS [15]. Absorbance of the samples at 278 nm was determined by UV/Vis spectrophotometry with pH 7.4 PBS as blank. The cumulative percent drug at various time intervals was calculated and plotted against time. All experiments were done in triplicate.
Results and Discussion
The amount of drug within respirable particles and percentage of the drug reaching the lung was according to characteristics of powder formulations. Drug deposition of DPI varies from 5-30% of the dose, but efficiency depends upon the device itself. A prominent part of breath actuated DPI was the yield of respirable particles (<5 μm diameter) from the device will proportional to airflow rate through the device. The key factor is rapid inhalation to deaggregate drug and carrier complexes or large agglomerates of drug particles. The motion of inhaled air supplies all of the energy associated with entrainment and deaggregation [16] of the powder particles. The powder was delivered during a single, large breath of the patient’s inspiration. The lung inhalation of dry powder inhaler required dispersion of powder particles into the individual particles. This was attained by adhesive forces between particles in the bulk powder [17]. The prediction of adhesive forces is not possible in pharmaceutical dry powder particles. The deposition of whole dose of DPI in lung was accessed with maximal and submaximal inspiratory effort and thus causes particle detachment from the carrier [18].
The particle size, and entrapment efficiency were evaluated for microparticles as given in Table 3. The mean particle size of microparticles ranged from 4.79 to 5 μm of all four trials. The size distribution of microparticles (laser diffraction) exhibited slight variation with different drug:polymer ratios of trials K1-K4. The <5 μm particle size of microparticles were achieved with an application of adequate energy given during processing time. Entrapment efficiency found to be 77-78% for all four trials varied slightly with the various proportion of polymers. The processing method and energy applied in the preparation of microparticles were controlled and achieved, the lesser deviation with entrapment efficiency. The Table 4 described on the weight variation and assay of DPI capsule contain SS microparticles and lactohale mix of trials K1-K4. The average weight of filled capsules was 20 mg containing 1 mg of SS microparticles. In all formulation assessment confirms the uniformity of drug content in the capsules. These results demonstrated that the right calculated quantity of salbutamol available in DPI trials K1-K4.
| Formulationcode | Drug:polymera | Mean±SD | |
|---|---|---|---|
| Particle size (D0.5 µm) | Entrapmentefficiency percentage | ||
| K1 | 1:1 | 5.02±0.6 | 78.70±0.06 |
| K2 | 1:2 | 4.69±0.7 | 76.50±0.04 |
| K3 | 1:3 | 4.86±0.4 | 77.30±1.20 |
| K4 | 1:4 | 4.79±0.3 | 77.60±1.84 |
SD: Standard deviation
Table 3: Physical characteristics of microparticles
| Formulation code | Weight variation (mg±SD) | Drug content percentage |
|---|---|---|
| K1 | 19.99±0.08 | 98.2–101.3 |
| K2 | 20.03±0.12 | 99.5–102.5 |
| K3 | 20.15±0.20 | 98.5–101.1 |
| K4 | 20.09±0.17 | 99.9–100.8 |
The values are represented as mean±SD for n=20. SD: Standard deviation
Table 4: Physicochemical haracterization of dry powder inhaler capsules
FTIR spectroscopy was used in compatibility studies to evaluate preformulation characteristics including formulation composition and poor interaction between carrier molecules. Salbutamol sulphate was determined to be stable with the formulation of trials K1, K2, K3 and K4 from the observation of FTIR spectra shows wave number (cm-1) vs Transmittance (%).
Fourier transform infrared spectroscopy (FT-IR) was performed on pure drug and formulation K1, K2, K3 and K4 and fig. 1a-e, shows the FT-IR of salbutamol sulphate and formulation respectively. No difference in the positions of absorption bands was observed in the spectra of salbutamol sulphate and formulations, indicating no chemical interaction between drug and polymer in solid state. Spectra of salbutamol sulphate showed sharp bands at wavelength of about 1000 cm-1, while spectra of formulation also show bands at same wavelength, but somewhat less intense due to drug polymer complex. This indicated less probability of chemical interaction of drug with other excipients. SEM of the formulations (K1-K4) is shown in fig. 2a-d, respectively. All the microspheres were smooth almost spherical in shape and non porous.
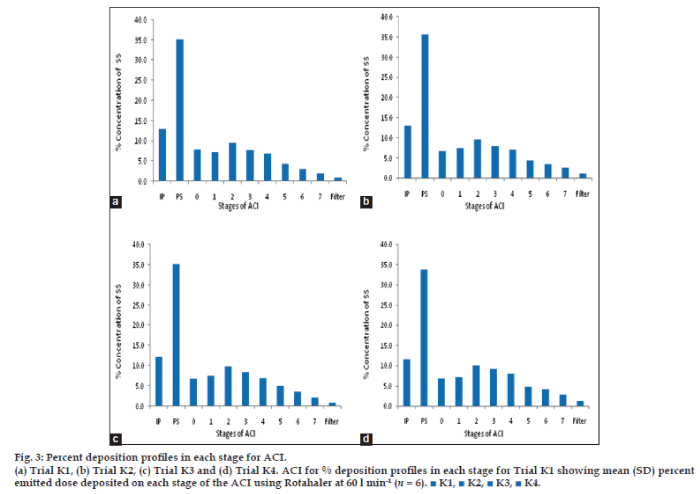
The In vitro deposition [19] of powder particle in the lung were evaluated by powder particle distribution in the stages of Anderson cascade impactor. The distribution of powder (microsphere) were graphically demonstrated in fig. 3a-d. The respiratory fraction and mass median aerodynamic diameter [20,21] of trial K1-K4 were given in Table 5.
Fig. 3: Percent deposition profiles in each stage for ACI.
(a) Trial K1, (b) Trial K2, (c) Trial K3 and (d) Trial K4. ACI for % deposition profiles in each stage for Trial K1 showing mean (SD) percent emitted dose deposited on each stage of the ACI using Rotahaler at 60 l min-1 (n = 6). ■ K1, ■ K2, ■ K3, ■ K4.
| Particle size Attributes | ACI (inhalation indices) mean±SD | |||
|---|---|---|---|---|
| K1 | K2 | K3 | K4 | |
| Emitted dose (%) | 91.2±2.52 | 90.2±3.12 | 89.6±2.37 | 94.3±2.85 |
| Respiratory fraction (%) | 34.5±3.21 | 35.8±3.10 | 36.9±3.31 | 39.7±3.36 |
| Total recovery | 96.7±2.48 | 97.6±2.92 | 98.1±2.86 | 98.5±1.92 |
| Mean median aerodynamic diameter (μm) |
2.72±0.01 | 2.81±0.01 | 2.89±0.01 | 2.32±0.01 |
Results for the trial K1–K4 measured using an air-flow rate of 60 L/min (n=6). Respiratory fraction calculated as ratio of total drug deposited in the lower stages of the Anderson cascade impactor (stages 2–8) to total theoretical dose. SD: Standard deviation, ACI: anderson cascade impactor
Table 5: Anderson cascade impactor results for the trials k1-k4
The dissolution was carried out in PBS at pH 7.4 at 37°±0.5°. The drug released at 12 h of formulation trials were as follows: K1, 92.57%; K2, 81.92%; K3, 78.42%; and K4, 77.71%. The drug releases were found to be not less than 77% at 12 h in all trials. The fig. 4 represents, cumulative percent drug release versus time in hours for trials K1-K4. It was understood that the drug release versus time was the linear curve and shows sustained release of SS in all formulation trials of K1 to K4. Among the four trials, formulation K1 exhibit better drug release over other trials K2-K4. Thereby 1:1 ratio of drug:polymer exhibited as best dissolution result.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- Guinness AE. The respiratory system. In, Cox J. A-Z of the human body, 1st ed. New York: Reader's Digest Association; 1987, p. 122-3.
- McConnell W, Holgate ST. The definition of asthma: its relationship to other chronic obstructive lung diseases. In: Clark TJH, Godfrey S, Lee TH, Thomson NC, editors. Asthma. 4th ed. London: Arnold; 2002. p. 1-5.
- The respiratory system. In Wilson KJW, Ross JS editors. Ross and Wilson's Anatomy and physiology health and illness. 7th ed. London: Churchill Livingstone; 1990. p. 138-40.
- Pharmacotherapy of Bronchial Asthma and Rhinitis. In: Satoskar RS, Bhandarkar SD, Ainapure SS. Pharmacology and Pharmacotherapeutics14th ed. Mumbai: Popular Prakashan; 1994. p. 313-20.
- Finlay WH. Dry powder inhalers. In, Finlay WH, editor. The Mechanics of Inhaled Pharmaceutical Aerosols, An Introduction. 1st ed. London: Academic Press; 2001. p. 221-73.
- Dandagi PM, Mastiholimath VS, Gadad AP, Iliger SR. Mucoadhesive microspheres of propanolol hydrochloride for nasal delivery. Indian J Pharm Sci 2009;69:402-7.
- Zeng XM, Martin GP, Marriotte C. The controlled delivery of drugs to the lung. Int J Pharm 1995;124:149-64.
- Hardy JG, Chadwick TS. Sustained release drug delivery to the lungs: An option for the future. ClinPharmacokinet 2000;39:1-4.
- Cook RO, Pannu RK, Kellaway IW. Novel sustained release microspheres for pulmonary drug delivery. J Control Release 2005;104:79-90.
- Morde MA, Bajaj AN, Bhanushali RS. Rifampicin pulmospheres for pulmonary delivery. Indian J Pharm Sci 2009;71:718-21.
- Mayur K, Padhi BK, Chougule M, Mishra A. Methotrexate loaded solid lipid nanoparticles for topical treatment of psoriasis: Formulation and clinical implication. Drug DelivTechnol 2002;5:1-13.
- Reddy LH, Murthy RS. Etoposide-loaded nanoparticles made from glyceride lipids: Formulation, characterization, in vitro drug release, and stability evaluation. AAPS PharmSciTech 2005;6:E158-66.
- Hasçiçek C, Gönül N, Erk N. Mucoadhesive microspheres containing gentamicin sulfate for nasal administration: Preparation and in vitro characterization. Farmaco 2003;58:11-6.
- Hassan MS, Lau R. Inhalation performance of pollen-shape carrier in dry powder formulation with different drug mixing ratios: Comparison with lactose carrier. Int J Pharm 2010;386:6-14.
- Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm 2003;255:175-87.
- Israelachvili JN. A review of intermolecular and surface forces. J Dis SciTechnol 1992;13:718-9.
- Pitcairn GR, Lankinen T, Seppälä OP, Newman SP. Pulmonary drug delivery from the Taifun dry powder inhaler is relatively independent of the patient’s inspiratory effort. J Aerosol Med 2000;13:97-104.
- Parmar JJ, Singh DJ, Lohade AA, Hegde DD, Soni PS, Samad A, et al. Inhalational system for etoposide liposomes: Formulation development and in vitro deposition. Indian J Pharm Sci 2011;73:656-62.
- Chan HK. Dry powder aerosol delivery systems: Current and future research directions. J Aerosol Med 2006;19:21-7.
- Stainforth JN. Performance modifying influences in dry powder inhalation systems. Aerosol SciTechnol 1995;22:346-53.
- Sunitha R. Drug delivery and its developments for pulmonary system. Int J Pharm Chem Bio Sys 2011;1:66-82.