- *Corresponding Author:
- S. C. Park
College of Veterinary Medicine, Kyungpook National University, Daegu 702-701, Republic of Korea
E-mail: parksch@knu.ac.kr
| Date of Submission | 27 May 2017 |
| Date of Revision | 05 April 2018 |
| Date of Acceptance | 12 October 2018 |
| Indian J Pharm Sci 2018;80(6):1108-1114 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Rifaximin, a virtually non-absorbed rifamycin drug is gaining attention for its broad-spectrum of activity. A couple of methods have been reported for its determination in biological matrices. However, inconvenient sample preparation procedure, lack of sensitivity and long analysis time hindered the application of those methods in pharmacokinetic and toxicological study. Thus, a high performance liquid chromatography method has been developed and validated in this study for the sensitive quantification of rifaximin in serum. In this procedure, chromatographic separation was achieved by flowing a mobile phase comprise of acetonitrile and 0.1 % acetic acid (60:40, v/v) through a C18 column (150×4.6 mm, 5 μm). An ultraviolet detector was utilized to identify and quantify the compound. The method has been validated over a concentration range of 0.03-30.00 μg/ml (r2=0.9999) by utilizing 150 μl of serum. The accuracy of the method was 89.59-98.84 %, and the intra- and inter-day precisions were 0.41-6.84 and 1.83-5.71 %, respectively. The compound was stable in different storage conditions. Insignificant amounts (2.40±0.38 to 4.22±1.10 μg/ml) of rifaximin were quantified in serum samples of rat from 2 to 8 h after oral administration. This method offers a rapid, sensitive, specific, reproducible, and stable tool for the quantitative determination of rifaximin in serum, which could be applicable in other biological matrices and species for pharmacokinetic and toxicological study of rifaximin.
Keywords
Rifaximin, hepatic encephalopathy, serum content, HPLC method, method development, validation
Hepatic encephalopathy (HE) is the second most common major complication in cirrhosis following ascites[1]. It is a complex neuropsychiatric syndrome characterized by a general depression of the central nervous system, with clinical manifestations ranging from only minor signs of altered brain function, overt psychiatric and/or neurological symptoms to deep coma, commonly reversible after therapy[2]. Treatment of an acute episode of HE consists of both the removal of any precipitating event, such gastrointestinal bleeding, constipation, electrolyte imbalance and infection, and in lowering ammonia production in the bowel, by using cathartic procedures and nutritional support[2,3]. Nonabsorbed disaccharides such as lactulose and lactitol are widely used as first-line drugs for the treatment of HE. However, long-term treatment of HE with disaccharides is not only ineffective but also initiates adverse effects in patients. Non-absorbable antibiotics are generally used as an alternative to disaccharides for the treatment of HE[4,5].
Rifaximin (Figure 1) is one such non-absorbable antibiotic that is derived from a semisynthetic derivative of rifamycin. It is a gastrointestinal-selective antibiotic with a broad spectrum of antimicrobial activity, excellent safety profile, minimal drug interactions, and negligible impact on the intestinal microbiome[6]. This chemical compound possesses its antibacterial activity in Gram-negative, Gram-positive, aerobic, and anaerobic bacterial classes and causes a little or no incidence of bacterial resistance[7]. Rifaximin is currently approved in the United States for the treatment of travellers’ diarrhoea caused by noninvasive diarrheagenic Escherichia coli[6] and is approved in more than 30 other countries for a variety of gastrointestinal disorders[8]. This antibacterial agent may also be applicable in the eradication of invasive enteric pathogens in the gastrointestinal tract prior to mucosal penetration[9]. The established clinical indications of rifaximin include infectious diarrhoea, HE, small intestinal bacterial overgrowth, colonic diverticular disease, and inflammatory bowel disease[10]. These diversified clinical applications of rifaximin demands the availability of analytical techniques for its precise, rapid and simple determination in biological matrices.
However, the determination of rifaximin in biological matrices is quite difficult due to its complexity and the existence of a number of endogenous elements. Sample clean-up processes also play a critical role in eliminating the overlapping of endogenous substances with the peaks of interest. Few methods including spectrophotometric, reversed-phase high-performance liquid chromatography (RP-HPLC), and liquid chromatography-mass spectrometry[11-14] have been described recently to analyse rifaximin. However, these approaches are not suitable and sensitive enough for the quantification of rifaximin from a complex sample matrix. A simple and highly sensitive method is necessary for the determination of pharmacological and toxicological aspects of this drug in biological system. This requirement encouraged us to develop and validate a simple and sensitive quantification technique for determining the serum concentrations of rifaximin.
The objective of the present study was to develop a method for highly sensitive, accurate and rapid quantification of rifaximin in serum and to apply the method in pharmacokinetic determination of rifaximin in rats. The sample clean-up step is not needed in this method. Rifaximin can quantifiable in nano-gram level from serum samples by using this simple and cost effective method, and the method could be highly applicable for toxicological and pharmacokinetic determinations.
Materials and Methods
Refaximin powder and HPLC grade acetic acid were purchased from Sigma-Aldrich (St. Louis, MO 63103, USA). HPLC grade acetonitrile and methanol were obtained from Honeywell Burdick & Jackson (Ulsan 680-160, Korea). De-ionized water was purified using a Milli-Q system (Millipore, Bedford, MA 01730, USA).
Hewlett-Packard Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA 95051, USA) equipped with a G1322A degasser, a G1311A quaternary pump, a G1313A auto-sampler, a G1316A column compartment, and a G1314A UV detector was used. Agilent ChemStation software (Agilent Technologies, Santa Clara, CA 95051, USA) was used to perform the chromatographic analysis. The 5 μm C18 column with, 150 mm length and 4.6 mm inner diameter (Fortis Technologies Ltd., Cheshire, CH64 3UG, United Kingdom) was used for the separation of compound. The column was conditioned prior to its first use and at the beginning of each day according to the recommended protocol. Nylon syringe filter (Thermo Scientific, Rockwood, TN 37854, USA) and microspine filter tubes (Sigma-Aldrich, St. Louis, MO 63103, USA) of 0.45 μm porosity were used to filter the standard and sample solutions respectively, prior to inject them in HPLC system.
Chromatographic conditions
The mobile phase was a mixture of acetonitrile and 0.1 % acetic acid with a ratio of 60 and 40 parts, respectively. The flow rate of the mobile phase was 1 ml/min and the column compartment was maintained at 30°. The injection volume of the filtered standard and sample solutions were 50 μl. The UV detection of the compound was done at a wavelength of 237 nm. The run time for one sample was 10 min.
Refaximin standard solution preparation
Stock standard solution of refaximin with a concentration of 10 mg/ml was prepared by dissolving 100 mg refaximin powder (Sigma-Aldrich, St. Louis, MO 63103, USA) with 10 ml of 100 % HPLC grade methanol (Honeywell Burdick & Jackson, Ulsan 680-160, Korea). The stock solution was then diluted with 100 % methanol to prepare a series of rifaximin working standards ranging from 0.03-30.00 μg/ml. The solution was filtered through a syringe filter of 0.45 μm pore and collected in a vial. Every day, the stock and working standard solutions were prepared freshly. Fifty microliter standard solutions of each concentration were injected to the HPLC system.
Refaximin-spiked serum sample preparation
Rifaximin stock solution (10 mg/ml) was diluted with 100 % methanol to prepare a series of rifaximin solutions ranging from 0.06-60.00 μg/ml. Serum previously collected from healthy rats housed in controlled environment with feeding of standard chow pellets and water ad libitum, was preserved in a –20° refrigerator. Rifaximin solutions (150 μl) of each concentration were separately taken in Eppendorf tubes with 150 μl serum, and mixed properly by vortexing. The tubes were centrifuged at 12 000 rpm for 5 min. The supernatants were transferred to a micro spine filter tube and centrifuged again at 12 000 rpm for 10 min. The filtrate was collected in a vial and injected to the HPLC system.
Validation of analytical method
The linearity of an analytical method is the capability of this method to elicit test results, which are directly proportional to the concentration of analytes in samples within a given range. Linearity was accomplished by a series of 6 injections from each concentration of rifaximin-spiked serum samples (6 different concentrations) ranging from 0.03- 30.00 μg/ml. The linear regression was determined by plotting the concentrations (x-axis) of rifaximin against the respective peak areas (y-axis). The linear relationship between concentration and peak area was established by a value of correlation coefficient (r2) which was greater than 0.99. The accuracy of an analytical method is the extent to which test results generated by the method and the true value of analytes. The precision of a method is the extent to which the individual test results of multiple injections of a series of standards agree. Each of the 4 different concentrations (0.03, 0.30, 3.00, 30.00 μg/ml) of rifaximin-spiked serum sample were separately injected six times to the HPLC system to determine the accuracy and precision of the analysis method.
Recovery is stated by means of the amount of analyte found in spiked sample as a percentage to the theoretical amount thought to be present in the medium. The absolute recoveries of rifaximin from spiked samples were evaluated in 4 different concentrations with six injections from each concentration. The lower limit of detection (LOD) is defined as the lowest concentration of analyte in a sample that can be detected, but not necessarily quantitated, under the stated experimental conditions. The lower limit of quantification (LOQ) is also similarly defined but as the lowest concentration of analyte in a sample that can be quantifiable under the stated experimental conditions. LOD and LOQ are practically determined by repeatedly injecting different lowest concentrations of rifaximin standard solutions. LOD can be calculated from the standard deviation of responses and the slope obtained from the calibration curve as stated by the Eqn., LOD = (3.3×SD)/slope[15,16]. LOQ can also be calculated from the standard deviation of responses and the slope associated with the calibration curve, but according to the following Eqn., LOQ = (10×SD)/slope[15,16]. The specificity of the method was ascertained by analysing the standard compound in standard and spiked solutions. The peaks for rifaximin in the spiked samples were confirmed by comparing the retention times of the sample peak with that of the standard. The peak purity of this compound was assessed by comparing the spectra at two levels, viz; peak start (S) and peak end (E) positions.
Application of the method to pharmacokinetic studies
In this study, 10 rats (180-200 g) which were housed in controlled environment with feeding of standard chow pellets and water ad libitum were used. A single dose of 20 mg/kg of rifaximin was administered orally to healthy rats and blood samples were collected at regular intervals of time for 24 h. The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Kyungpook National University (approval number KNU 2011-1). Serum was separated by centrifugation at 4500 rpm for 10 min and stored at −20° until analysis. They were centrifuged at 12 000 rpm for 5 min. The supernatants were transferred to a micro spine filter tube and centrifuged again at 12 000 rpm for 10 min. The filtrate was collected in vial and injected to the HPLC system.
Data analysis
The data were analysed by using Microsoft Excel 2010 statistical package. The standard deviations, standard errors, and linear regression were calculated from peak area values of six different samples.
Results and Discussion
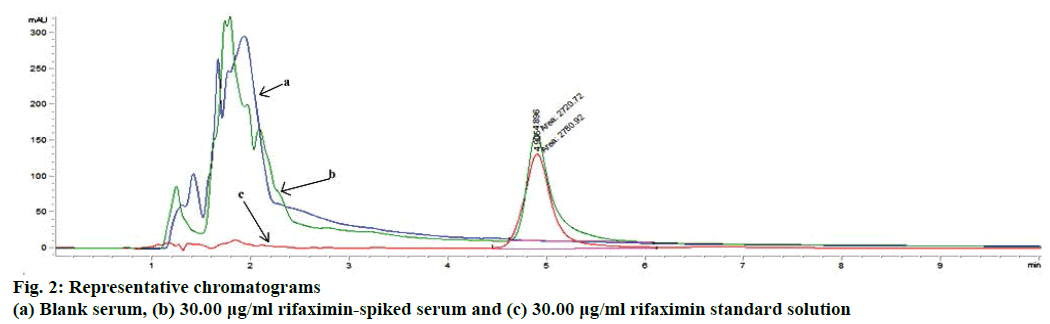
In the current study a HPLC method was developed in order to identify and quantify the rifaximin from biological matrix. Generally, it requires several trials to select a suitable mobile phase for the development of a method; because of the complexity and composition of biological samples and the affinities of the components towards various solvents. Different reversed-phase columns (C8 and C18) as a stationary phase with several mobile phase compositions using various flow-rates (0.50-1.50 ml/min) were utilized in this investigation to get improved chromatographic conditions including better resolution, better symmetry and minimized tailing of rifaximin peak with a faster retention time. The best separation and intense peak of rifaximin was achieved by using C18 column (5 μm, 150 mm length and 4.6 mm inner diameter) with a mobile phase of acetonitrile and 0.1 % acetic acid at 60:40 (v/v) ratios by a flow rate of 1.0 ml/min. The detection wavelength was determined as 237 nm by using ultraviolet detector. When the standard and spiked serum solutions were injected 6 times separately, the retention times of rifaximin were found to be the same (Figure 2). The developed chromatographic method was validated according to “International Conference on Harmonization (ICH) guideline for the validation of analytical procedures”[16] in respect to the selectivity of method, linearity, LOD, LOQ, accuracy, precision, recovery, and robustness.
Linearity of a method is the ability to produce test results that are directly proportional to the concentrations of the analyte within a given range, and linear responses were established from the concentration verses response curve for rifaximin in this study. The acceptance criterion for linearity is that the correlation coefficient (r2) should not be less than 0.99 for the least squares method of the analysis of the line[16]. The correlation coefficient, slope, and intercept of rifaximin in this study were 0.9999, 91.129 and –8.2906, respectively. This result demonstrates the linearity of this method over a wide dynamic range. The recovery of rifaximin from spiked sample is shown in Table 1, which were determined at four different concentrations by comparing the mean of peak areas. The mean recoveries of rifaximin in serum matrix were 89.59-98.84 % (Table 1). The recovery percentages of this compound in different concentrations of samples are adequate. The expected recovery is dependent on the percentage of analyte in matrix[17] indicating that, the analytical method is validated.
| Concentration (μg/ml) | % Recovery |
|---|---|
| 0.03 | 89.61 ± 1.32 |
| 0.30 | 89.59 ± 2.00 |
| 3.00 | 91.31 ± 1.92 |
| 30.00 | 98.84 ± 0.41 |
Table 1: Percent Recovery of rifaximin from rat serum
Accuracy and precision were determined from four different concentrations of rifaximin-spiked serum samples by calculating the percent of recovery and percent of relative standard deviation (% RSD), correspondingly, for each set of test samples. Table 2 exhibits the accuracies, intra-day precision (repeatability), and inter-day precision (reproducibility) of the analytical method. The accuracies of the analysis method attained for rifaximin were 89.59-98.84 % in spiked samples. The intra-day and inter-day precision of this assay method were within the limit for all tested concentration according to the guidelines for analytical method development and validation[17,18]. The % RSD and standard error are within the limits, which indicate that, the assay method is validated considering precision.
| Concentration (μg/ml) | Intra-day | Inter-day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||||||||||
| Accuracy | % RSD | SE | Accuracy | % RSD | SE | Accuracy | % RSD | SE | Accuracy | % RSD | SE | |
| 0.03 | 89.61 | 1.47 | 0.85 | 86.90 | 6.73 | 3.89 | 89.81 | 2.47 | 1.43 | 88.77 | 1.83 | 1.06 |
| 0.30 | 89.59 | 2.23 | 1.29 | 86.72 | 6.84 | 3.95 | 96.82 | 2.89 | 1.67 | 91.04 | 5.71 | 3.30 |
| 3.00 | 91.31 | 2.11 | 1.22 | 94.51 | 2.56 | 1.48 | 98.28 | 2.16 | 1.25 | 94.70 | 3.68 | 2.13 |
| 30.00 | 98.84 | 0.41 | 0.24 | 96.21 | 0.56 | 0.32 | 100.65 | 1.14 | 0.66 | 98.57 | 2.27 | 1.31 |
Table 2: Analysis of rifaximin-spiked serum samples
According to ICH guideline[19], there are several approaches to determine the LOD and LOQ. Visual evaluation, signal-to-noise ratio, and the use of standard deviation of the response and the slope of the calibration curve are generally used methods. The LOD and LOQ in the present study were determined based on the last approach. The LOD was 0.009 μg/ml for rifaximin, which means that peak areas for these concentrations should be distinguishable from the response given by blank sample. The LOQ of rifaximin was 0.03 μg/ml, therefore, the compound can be reliably quantifiable at concentrations equal to, or higher than 0.03 μg/ml.
Specificity of the analytical method ensures that the signals measured come from the desired compounds and there is no interference from diluents, endogenous compounds of biological samples and mobile phase. The ultraviolet detection supported the specificity of the method and provided evidence for the homogeneity of the peak of analyte (Figure 2). Peaks obtained from recovery experiments were checked for uniformity using UV spectra taken from different points of the peak of interest. These UV spectra were superimposed whenever overlaid; showing that there was no other coeluting peaks, in every instance for each of the analytes. The data obtained in the validation study proved that the proposed method is validated and can be utilize for the determination and quantification of rifaximin in serum samples.
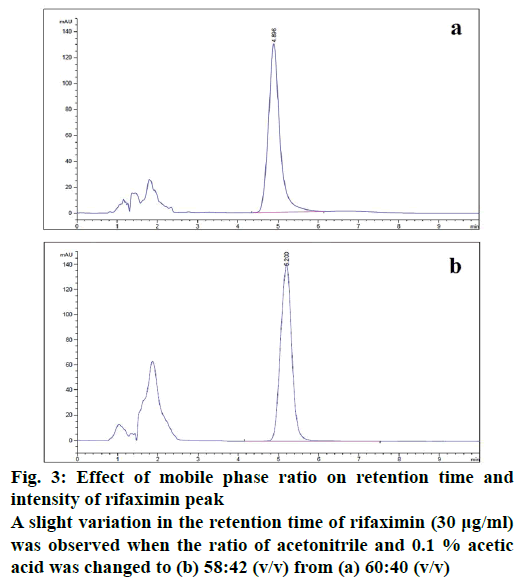
The developed method was checked for robustness by slightly changing some method settings. A small change in the ratio of mobile phase solvents (acetonitrile and 0.1 % acetic acid) only shifted the retention time to some extent without affecting the resolution (Figure 3). It showed a minor variation in retention time and resolution with the slight variation of flow rate. The temperature of the column compartment was also changed at 30 ± 2° and observed an insignificant variation in the resolution.
The stability studies of rifaximin were executed by determining the concentration differences of the compound in freshly prepared and post-prepared serum samples kept at different storage conditions for a designated period of time. Four different concentrations of samples were prepared, and evaluated the short and long term stability. The stability of the compound at room temperature was assessed after 24 h of sample preparation and observed that the compound is stable up to that time point with a negligible variation. Serum samples of rifaximin stored at −20° refrigerator were analysed after 3, 7, 15 and 30 d of sample preparation for the determination of long term stability. The compound in the serum matrix was stable up to 30 d of post-preparation and the measured concentrations of rifaximin in different samples stored at different conditions are presented in Table 3. These results indicate the adequate stability of rifaximin in serum sample for long time and suitable for the functional use.
| Theoretical Concentration (μg/ml) | Measured Concentration (μg/ml) | |||||
|---|---|---|---|---|---|---|
| Day 1* | Day 2** | Day 3 | Day 7 | Day 15 | Day 30 | |
| 0.03 | 0.027 ± 0.001 | 0.027 ± 0.000 | 0.027 ± 0.001 | 0.027 ± 0.000 | 0.026 ± 0.002 | 0.027 ± 0.001 |
| 0.30 | 0.290 ± 0.008 | 0.289 ± 0.004 | 0.289 ± 0.003 | 0.308 ± 0.022 | 0.260 ± 0.018 | 0.303 ± 0.026 |
| 3.00 | 2.948 ± 0.064 | 2.936 ± 0.070 | 2.960 ± 0.079 | 2.739 ± 0.058 | 2.835 ± 0.073 | 2.933 ± 0.062 |
| 30.00 | 30.196 ± 0.346 | 30.044 ± 0.395 | 30.071 ± 0.161 | 29.653 ± 0.123 | 28.863 ± 0.162 | 30.337 ± 2.180 |
Table 3: Stability of freeze-thawed rifaximin-spiked serum samples
In the initial hour rifaximin was not identified from serum sample of rat. Small absorbance of rifaximin was found in biological matrixes from 2 to 8 h. The concentrations of rifaximin in serum samples were 2.75 ± 0.49, 3.10 ± 0.68, 2.40 ± 0.38 and 4.22 ± 1.10 μg/ml, respectively at 2, 4, 6 and 8 h. Refaximin was not identified from the serum samples of 12 h and later on. Negligible amount of drug compound was detected from the serum samples throughout the analysis period, which is justified with the poor absorption capability of rifaximin.
This study described the development and validation of a simple, precise, and highly sensitive HPLC method for the rapid determination of rifaximin in serum. The method is selective and its lower quantification limit is 0.03 μg/ml. The analysis time for each sample is less than the conventional HPLC method described for the quantification of rifaximin from biological fluid. A wide linearity range (0.03-30.00 μg/ml) was achieved in this method with good recoveries and reproducibilities. The analysis of rifaximin by this method requires only 150 μl of serum, which greatly facilitates the sample collection. This method could be utilized as a practical tool for pharmacokinetic and toxicological determinations of rifaximin.
Acknowledgements
This research was supported in part by Korean Institute of Planning and Evaluation for Technology (IPET) through Technology Commercialization Support Program (314082-3), and in part by The Technology Development Program for Forestry (S111515L050130), Korea Forest Service, and in part by the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01128901), Rural Development Administration, Republic of Korea.
Conflict of interests
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Gentilini P, Vizzutti F, Gentilini A, Zipoli M, Foschi M, Romanelli RG. Update on ascites and hepatorenal syndrome. Dig Liver Dis 2002;34(8):592-605.
- Riggio O, Ridola L, Pasquale C. Hepatic encephalopathy therapy: An overview. World J Gastrointest Pharmacol Ther 2010;1(2):54-63.
- Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol 2010;7:515-25.
- Adachi JA, DuPont HL. Rifaximin: A Novel Nonabsorbed Rifamycin for Gastrointestinal Disorders. Clin Infect Dis2006;42:541-7.
- Sama C, Morselli-Labate AM, Pianta P, Lambertini L, Berardi S, Martini G. Clinical effects of rifaximin in patients with hepatic encephalopathy intolerant or nonresponsive to previous lactulose treatment: An open-label, pilot study. Curr Ther Res Clin Exp 2004;65:413-22.
- Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol 2010;26:17-25.
- DeMarco F, Amato PS, D’Arienzo A. Rifaximin in collateral treatment of portal-systemic encephalopathy: A preliminary report. Curr Ther Res 1984;36:668-74.
- Alvisi V, D’Ambrosi A, Loponte A, Pazzi P, Greco A, Zangirolami A, et al. Rifaximin, a rifamycin derivative for use in the treatment of intestinal bacterial infections in seriously disabled patients. J Int Med Res 1987;15:49-56.
- DuPont HL. Systematic review: prevention of travelers’ diarrhea. Aliment Pharmacol Ther 2008;27:741-51.
- Scarpignato C, Pelosini I. Rifaximin, a Poorly Absorbed Antibiotic: Pharmacology and Clinical Potential. Chemotherapy 2005;51(suppl 1):36-66.
- Bidyut A, Amrutansu A, Annapurna MM. Spectrophotometric estimation of rifaximin in pure and tablet dosage form. Int J PharmTech 2010;2:1098-104.
- Kasimala BB, Syed R, Pammi K, Sandhya B. RP-HPLC method development and validation for the analysis of rifaximin in pharmaceutical dosage forms. IJRRPAS 2011;1:323-33.
- Sudha T, Hemalatha PV, Ravikumar VR, Jothi R, Radhakrishnan M. Development and validation of RP-HPLC method for the estimation of rifaximin in bulk and in tablet dosage form. AsianJ Pharm Clin Res 2009;2:112-6.
- Sudha T, Anandakumar K, Hemalatha PV, Ravikumar VR, Radhakrishnan M. Spectrophotometric estimation methods for rifaximin in tablet dosage form. Int J Pharm Pharm Sci 2010;2:43-6.
- ICH, Q1A, Stability Testing of New Drug Substances and Products. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf.
- ICH, Q2, Validation of Analytical Procedures: Text and Methodology. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- AOAC, Standard Format and Guidance for AOAC Standard Method Performance Requirement. Available from: http://www.eoma.aoac.org/app_f.pdf.
- FDA, Guidance for Industry: Bioanalytical Method Validation. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf.
- ICH, Q2B, Validation of Analytical Procedures: Methodology. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm073384.pdf.