- *Corresponding Author:
- Sivakumar T
Psychiatry Department at Yale Schol of Medicine Yale University, New Haven, CT 06519, USA
E-mail: tsivakumaar@yahoo.com
| Date of Received : | 21 February 2007 |
| Date of Revised : | 26 June 2006 |
| Date of Accepted : | 03-Sep-2012 |
| Indian J. Pharm. Sci., 2007, 69 (1): 154-157 |
Abstract
A new reversed-phase high performance liquid chromatography method was developed and validated for the simultaneous determination of losartan potassium and atenolol in tablets. The separation was achieved on Supelcosil ODS analytical column (25×0.46 cm, i.d., 5 µm) using acetonitrile and 25 mM potassium dihydrogen phosphate (45:55 v/v, pH 3.00±0.05) as mobile phase at a flow rate of 1.2 ml/min. Detection was carried out using a UV detector at 227 nm. The method was validated. The developed and validated method was successfully applied for the quantitative analysis of Losar beta® tablets. The total chromatographic analysis time per sample was about 6 min with atenolol, chlorzoxazone (internal standard) and losartan eluting at retention times of about 2.72, 4.89 and 5.61 min, respectively. The standard curves were linear over the concentration ranges, 1 to 10 µg/ml for losartan potassium and atenolol. The values obtained of LODs were 0.029 and 0.062 µg/ml and LOQs were 0.078 and 0.187 µg/ml for losartan potassium and atenolol, respectively. The proposed method is fast, accurate and precise for the determination of losartan potassium and atenolol for routine quality control of tablets containing these two drugs.
Losartan potassium, a potassium salt of 2-Butyl-4-chloro-1[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1Himidazole-5-methanol (fig. 1), represents the first of a new class of orally active non-peptide angiotensin II (Type AT1) receptor antagonists employed in the management of essential hypertension [1-2]. The individual determination of losartan has been carried out in tablets by HPLC, capillary electrophoresis and super-critical fluid chromatography [3], in bulk and solid dosage forms by colorimetric method [4], simultaneously with its degradates in stressed tablets by LC-MS/MS [5] and HPTLC [6] and with its active metabolite in biological fluids by HPLC [7-9] .
Atenolol, 4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy] benzeneacetamide (fig. 1) is a selective ß1-adrenoceptor antagonist [10] applied in the treatment of numerous cardiovascular disorders such as hypertension and angina pectoris. Several analytical methods reported for the quantitative determination of atenolol individually in pharmaceutical formulations or in biological fluids, are HPLC [11-13], gas chromatography [14], capillary zone electrophoresis [15], titrimetry [16] and spectrophotometry [17] .
In recent years, these two drugs are successfully used in association in the treatment of hypertension and the pharmaceutical preparations containing both drugs have been marketed, permitting once a day administration. Although, many methods have been reported in the literature for the estimation of losartan potassium and atenolol individually, there is no single method reported for its simultaneous estimation. However, as the use of this preparation is increasing rapidly, it is very much essential to develop a suitable analytical method for the simultaneous estimation of losartan potassium and atenolol in its pharmaceutical forms. Such method should be simple, rapid, accurate and precise to be applied for routine quality control analysis, dissolution or similar studies. Hence, in the present study, a new reversed-phase high performance liquid chromatography method was developed and validated for the simultaneous determination of losartan potassium and atenolol in tablets.
Losartan potassium and atenolol were kindly gifted by Alembic Chemical Works, Vadodara, India. Chlorzoxazone (internal standard) was purchased from Madras Medical Company, Chennai, India. The commercially available Losar beta® tablets (containing losartan potassium-50 mg and atenolol-50 mg) were obtained from Unichem Laboratories Ltd., Mumbai, India. Acetonitrile and methanol were of HPLC grade while buffer salts and all other reagents employed were of analytical reagent grade supplied by M/S S. D. Fine Chemicals, Mumbai, India. The HPLC grade water was prepared by using Milli-Q Academic, Millipore, Bangalore, India.
The analysis were performed on a Shimadzu chromatographic system (Japan), equipped with an LC-10 ADvp solvent delivery Module, SPD-10A UV-Visible detector, and a Rheodyne model 7125 injector valve fitted to a 20 μl volume sample loop. Losartan potassium, atenolol and chlorzoxazone (internal standard) were resolved on a Supelcosil ODS analytical column (25×0.46 cm i.d., 5 μm) in the reversed-phase partition chromatographic condition. The mobile phase composed of acetonitrile and 25 mM potassium dihydrogen phosphate (45:55 v/v, pH 3±0.05). The flow rate was 1.2 ml/min and the analytes and internal standard monitored at 227 nm. The HPLC system was controlled by a PC workstation with Shimadzu chromatographic software (CLASS LC 10, Ver.1.63) installed. The system was used in an air-conditioned HPLC laboratory atmosphere (20±2°). Before the analysis, the mobile phase was degassed using Branson sonicator (Branson Ultrasonics Corporation, USA) and filtered through a 0.2 μm membrane filter (Gelman Science, India). The system was equilibrated before making injection.
Stock solutions of losartan potassium, atenolol and chlorzoxazone (IS) were prepared individually by dissolving accurately weighed 10 mg of the drug in 10 ml of methanol. The prepared stock solution was stored at 4° protected from light. From this stock solution standard 100 μg/ml was freshly prepared on the day of analysis.
Calibration standards for both the analytes were prepared individually from the standard solution of 100 μg/ml by appropriate dilution with mobile phase by varying the losartan potassium concentration in the range 1, 2, 5, 7 and 10 μg/ml (maintaining atenolol at 5.0 μg/ml) and the atenolol concentration in the range 1, 2, 5, 7 and 10 μg/ml (maintaining losartan potassium at 5.0 μg/ml). An aliquot of the internal standard solution, after appropriate dilution, was added to each standard solution (final concentration, 2.5 μg/ml). For the preparation of calibration curve for each analytes, an aliquot of 20 μl of this calibration standard solutions were injected. Concentrations of 1, 5 and 10 μg/ml were taken as quality control (QC) samples.
For estimating the tablet dosage form, 20 tablets from a batch were randomly selected and powdered. Amount equivalent to 25 mg each of losartan potassium and atenolol from powdered formulation were accurately weighed and taken in a 25 ml volumetric flask; suitable quantity of IS was added (final concentration, 2.5 μg/ml) followed by 15 ml of methanol. The mixture was subjected to sonnication for 10 min for complete extraction of drugs, and the solution was made up to the mark with methanol. The solution was centrifuged at 4000 rpm for 10 min; the supernatant was taken and diluted with mobile phase (final concentration, 5 μg/ml for each drug) and 20 μl of this solution was injected for HPLC analysis.
Acetonitrile in different ratios (40 to 50%) n the mobile phase composed of 25 mM potassium dihydrogen phosphate at pH 3.00 were tested. The retention time of losartan potassium, atenolol and internal standard, chlorzoxazone decreased with increasing acetonitrile concentration in the mobile phase. The better separation and resolution of these drugs were obtained when the percentage of acetonitrile in the mobile phase was 45%, offering short run time of about 6 min.
The system suitability was assessed by six replicate analyses of the analytes at a concentration of 5.0 μg/ml. The % CV of peak area and retention time for both drugs is ≤2. The efficiency of the column as expressed by number of theoretical plates for the six replicate injections was 4478±0.8 (mean±% CV) and the USP tailing factor was 1.14±0.3 (mean±% CV) for atenolol. The number of theoretical plates and the USP tailing factor for losartan potassium were found to be 9412±1.2 and 1.17±0.4, respectively.
Linearity was demonstrated at concentrations from 1 to 10 μg/ml for both the analytes. The mean (±SD) regression equation from six replicated calibration curves of losartan potassium and atenolol were y=0.3713 (±0.0006)x+0.0074 (±0.0284) and y=0.1675 (±0.0009)x+0.0064 (±0.0916) respectively. The correlation coefficients (R2) were 0.9995 (±0.0031) for losartan potassium and 0.9997 (±0.0027) for atenolol, indicating a high degree of linearity for both losartan potassium and atenolol calibration curves. Limits of detection (LOD) and quantitation (LOQ) were estimated from the signal to noise ratio. The values obtained of LODs were 0.029 and 0.062 μg/ml and LOQs were 0.078 and 0.187 μg/ml for losartan potassium and atenolol, respectively.
The recovery was studied at concentrations of 50% (n=3), 100% (n=6) and 150% (n=3) of the target level in the tablet. In recovery studies, a known amount of analytes was spiked (dry addition) with a determined amount of placebo and the amount of each analyte recovered in relation to the added amount was calculated. The placebo used in this validation study contained the following inactive ingredients: lactose monohydrate, microcrystalline cellulose PH 101, corn starch, povidone, colloidal silicon dioxide, magnesium stearate and titanium dioxide. The recovery was found to be in the range of 99.95 to 100.42% which was well within the acceptance limit of 98 to102%.
Accuracy of the assay method was determined for both intra-day and inter-day variations using the replicate (n=6) analysis of the QC samples of 1, 5 and 10 μg/ml. Accuracy and precision calculated for the QC samples during the intra-day and inter-day run are given in Table 1. The intra-day accuracy ranged from -1.00 to 4.00% and precision from 0.741 to 1.040%. The inter-day accuracy ranged from 0.00 to 3.4% and precision from 1.335 to 1.881%. The intra-day and inter-day precisions were within the acceptance criteria of %CV ≤1.0 and ≤2.0, respectively.
| Attributes | Conc. (µg/ml) | Mean conc.±SD (µg/ml), % CV, % Bias | |
|---|---|---|---|
| Atenolol | Losartan | ||
| Intra-Day | 1 | 1.02±0.008, 0.784, 2.00 | 1.04±0.009, 0.865, 4.00 |
| 5 | 5.02±0.048, 0.956, 0.40 | 4.99±0.037, 0.741, -1.00 | |
| 10 | 10.08±0.076, 0.754, 0.80 | 10.10±0.105, 1.040, 1.00 | |
| Inter-Day | 1 | 1.01±0.019, 1.881, 1.00 | 1.00±0.018, 1.800, 0.00 |
| 5 | 5.12±0.091, 1.777, 2.40 | 5.17±0.069, 1.335, 3.40 | |
| 10 | 10.16±0.160, 1.575, 1.60 | 10.11±0.179, 1.771, 1.10 | |
SD representsstandarddeviation and CV representscoefficient of variationforeachstandardsolution (n=6).
Table 1: Accuracy and precision of hplcassay
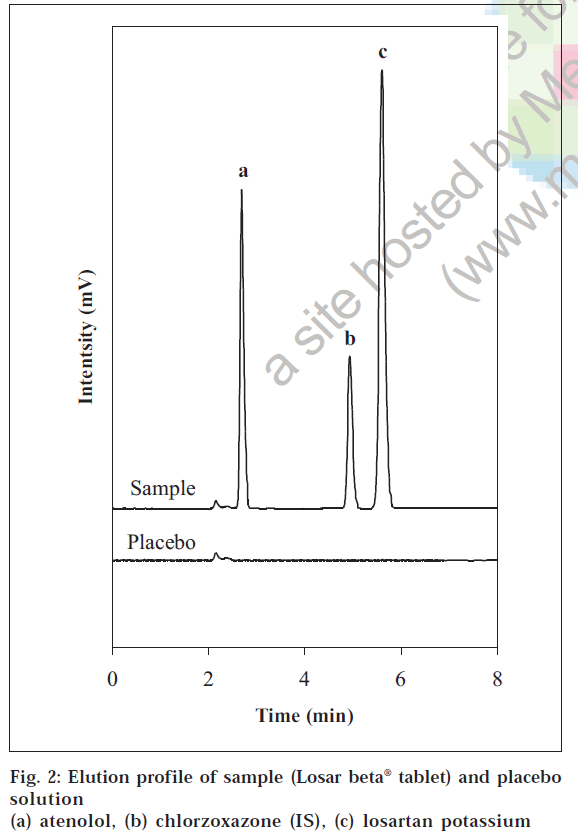
The specificity study revealed the absence of excipients (placebo) interference since none of the peaks appears at the same retention time of losartan potassium, atenolol and chlorzoxazone peak (fig. 2). Hence it was concluded that the developed method is selective in relation to the excipient used in this study.
The stability of the analyte solutions were determined using losartan potassium and atenolol standard solution (5 μg/ml) by keeping at ambient temperature for 48 h and analyzing. Stability studies indicated that the analytes were stable and are well within the acceptance level of 95% [18] .
The developed and validated HPLC method was applied for the determination of losartan potassium and atenolol content in a commercial antihypertensive tablet, Losar beta®. There was no interference of excipients in the
product examined, so no addition extraction or separation procedures are required during their assay. The total chromatographic run time per sample was 6 min with atenolol, chlorzoxazone (internal standard) and losartan eluting at retention times of about 2.72, 4.89 and 5.61 min, respectively (fig. 2). The % recovery (mean±S.D, n=6) was 100.02±0.21 for atenolol and 100.10±0.29 for losartan which were well within the acceptance limits of 95% to 105%.
The HPLC method described for the simultaneous determination of losartan potassium and atenolol has been found to be linear, precise, accurate and selective to be applied in routine and quality control analysis of tablets. In the proposed method, there are no additional extraction or separation procedures to extract the drug from the formulation excipient matrix thereby decreasing the error in quantitation.
References
- Smith, R.D., Chiu, A.T., Wong, P.C., Herblin, W.F. and Timmermans, P.B.M.W.M., Annu. Rev. Pharmacol. Toxicol., 1992, 32, 135.
- Chiu,A.T., McCall, D.E. and Price, W.A., J. Pharmacol. Exp. Ther., 1990, 252, 71l.
- Williams, R.C., Alasandro, M.S., Fasone, V.L., Boucher, R.J. and Edwards. J.F., J. Pharm. Biomed. Anal., 1996, 14, 1539.
- Prabhakar, A. H. and Giridhar, R., J. Pharm. Biomed. Anal.,2002, 27, 861.
- Zhao, Z.Z., Wang, Q., Tsai, E.W., Qin, X.Z. and Ip, D., J. Pharm. Biomed. Anal., 1999, 20, 129.
- McCarthy, K.E., Wang, Q., Tsai, E.W., Gilbert, R.E. and Brooks, M.A., J. Pharm. Biomed. Anal., 1998, 17, 671.
- Farthing, D., Sica, D., Fakhry, I., Pedro, A. and Gehr, T.W.B., J. Chromatogr. B, 1997, 704, 374.
- Ritter, M.A., Furtek, C.I. and Lo, M.W., J. Pharm. Biomed. Anal., 1997, 15, 1021.
- Yeung, P.K., Jamieson, A., Smith, G.J., Fice, D. and Pollak, P.T., Int. J. Pharm., 2000, 204, 17.
- Wadworth, A.N., Murdoch, D. and Brogden, R.N., Drugs, 1991, 42, 468.
- Pawlak, Z. and Clark, B.J., J. Pharm. Biomed. Anal., 1992, 10, 329.
- Terry, S. and Teitelbaum, Z., J. Liq. Chromatogr., 1991, 1, 3735.
- Lamprecht, G., Kraushofer, T., Stoschitzky, K. and Lindner, W., J. Chromatogr. B, 2000, 740, 219.
- Peng, J.H., Tu, J.S. and Xin, J.D., J. China Pharm. Univer., 1995, 26, 344.
- Shafaati, A. and Clark, B.J., J. Pharm. Biomed. Anal., 1996, 14, 1547.
- Ebeid, M.Y., Moussa, B.A., A-Maleck, A. and Ashour, F.M., EgyptJ. Pharm. Sci., 1997, 38, 171.
- Prasad, U.V., Rao, G.V. and Sastry, C.S.P., Indian J. Pharm. Sci., 2002, 64, 129.
- Lozano, P.P., Montoya, E.G., Orriols, A., Miñarro, M., Ticó, J.R. and Negre J.M.S., J. Pharm. Biomed. Anal., 2004, 34, 979.