- *Corresponding Author:
- Y.Mishra
School of Bioengineering and Biosciences, Lovely Professional University, Phagwara, Punjab 144411, India
E-mail: yachanamishra@gmail.com
| Date of Received | 21 September 2023 |
| Date of Revision | 20 March 2024 |
| Date of Acceptance | 19 July 2024 |
| Indian J Pharm Sci 2024;86(4):1517-1521 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The purpose of current research involved the development of a facile, precise, accurate, suitable, reproducible as well as robust ultraviolet spectroscopic method for assessment of paclitaxel. The spectrum of paclitaxel was scanned at 400-200 nm of range using a stock solution of paclitaxel (100 µg/ml) as reference solution and methanol:phosphate buffer solution (pH 7.4) (3:7) and methanol: phosphate buffer solution pH 7.4 (5:5) as blank. Linearity was assessed from the calibration curve prepared using solutions of concentration 2-20 µg/ml. The technique was validated by determining linearity, accuracy, precision, repeatability, limits of detection, limits of quantitation and ruggedness as per International Council for Harmonization Q2 (R1) guidelines. The absorbance maxima of paclitaxel was found to be 230 nm in the 2-20 µg/ml of range. The regression coefficient was found to be 0.9981 in methanol:phosphate buffer solution 7.4 (3:7) and 0.9976 in methanol:phosphate buffer solution 7.4 (5:5). Percent relative standard deviation of all specifications was found to be within the limits. This validated method can magnificently be executed in the assessment of paclitaxel.
Keywords
Paclitaxel, validation, ultraviolet-spectroscopy
Cancer report of World Health Organization (WHO) defines that cancer is the second greatest reason for deaths worldwide causing about 9.6 million deaths in 2018. Overall, the cause of 1 out of 6 deaths is cancer [1]. It depicts the uncontrolled growth of the cells. Several cancers are coined from a single cell that leads to preliminary mutat¬ions in which posterity endure auxiliary changes lacking several further mutations via transformation, normal cell changes into the cancerous cell which causes tumor [2]. National Center for Health Statistics gathered mortality data accessible through 2016 and projected that in 2019 about 1 762 450 new cases and 606 880 deaths associated with cancer will occur in the United States. However, as per 2006-2015 data, cancer death rate was constant in females and decreased (2 %) in males every year. While in 2007-2016 data, the death rate declined in women (1.4 %) and men in (1.8 %) annually. It is expected that if cancer death rate decreases continuously, still around 2 629 200 deaths will occur in coming years [3]. When the combination chemotherapy failed for treatment of metastatic breast cancer or in relapse during adjuvant chemotherapy of 6 mo, then Paclitaxel (PTX) is among the suggested regimens [4].
PTX, a di-terpenes pseudo-alkaloid derived from Pacific Yew (Taxus brevifolia) tree bark of Taxaceae family in 1971, is a tri- or tetracyclic 20 carbon chain skeleton compound [5,6]. The chemical structure of PTX is represented in fig. 1. PTX, one of the most vital and effectual anticancer bioactive, is regularly utilized in therapy of different types of cancers including lung, ovarian, brain, breast, head neck and acquired immunodeficiency syndromerelated Kaposi's sarcoma [6-8]. PTX was approved by United States Food and Drug Administration for clinical trials in 1992. The PTX falls under Class IV of Biopharmaceutics Classification System (BCS) showing both poor solubility and poor permeability.
The Mode of Action (MOA) of PTX is associated with selective tubulin polymerization and shielding of the cellular microtubule depolymerization, which consecutively inhibits tumor cell division [6]. PTX is predominantly metabolized in the liver by cytochrome (CYP) P450 enzymes. The primary metabolite 6-hydroxy-PTX (6-OH-PTX) is formed by CYP2C8, whereas the secondary metabolite 3-p-hydroxy-PTX (3-p-OH-PTX) is generated by CYP3A4 [9]. This mechanism has been applied successfully in cancer treatment in numerous preclinical and clinical trials [5,10]. The PTX administration is mainly linked with some of the adverse events including hematopoietic and neurologic toxicities. The inter-individual tolerability is partially associated with pharmacogenetic and pharmacokinetic dissimilarities among patients, especially in PTX clearance. The pharmacokinetics of PTX is non-linear because of saturable distribution and elimination of the drug. The metabolic clearance can be influenced by genetic polymorphisms, pathologic, demographic and physiologic factors as well as drug interactions [6].
PTX endorses microtubule assembly and impedes M-phase of the cell cycle or cellular replication in G2 phase. PTX is absorbed in the liver utilizing P450 isoforms, CYP3A4 and CYP2C8. It endures widespread vascular distribution and tissue binding. However, it never crosses Blood Brain Barrier (BBB) [11]. Several methods are available for estimation of PTX like high-performance liquid chromatography, mass spectrometry, dried blood spots based assay but these are somewhat complicated methods [6,9,12]. In this report we have designed and validated a simple, rapid, accurate, economic and precise method for the estimation of PTX.
PTX was received as a gift sample from Cipla India Pvt. Ltd. (Mumbai, India). A Ultraviolet (UV)- visible spectrophotometer (Shimadzu, model 1800, Kyoto, Japan) attached with computer software (UV-Probe), including two matched quartz cells and 1 cm light path was used. The instrument was tested for automatic wavelength accuracy, which was found to be 0.1. All analytical grade chemicals were used during this experiment. The Phosphate Buffer Saline (PBS) was prepared by dissolving 8g of sodium chloride, 2.38 g of disodium hydrogen phosphate and 0.19 g of dibasic sodium phosphate in 1000 ml of distilled water and adjusted the pH 7.4. The standard for choosing specific media was solubility of the drug, cost of solvents, sensitivity of the method, applicability as well as robustness.
PTX is found to be freely soluble in methanol and practically insoluble in PBS 7.4. In this regard analysis of PTX was accomplished by selecting 2 solvent systems consisting of methanol and PBS 7.4 in ratio of 3:7 and 5:5, respectively [13]. Accurately weighed 10 mg of PTX and transferred to a dry and clean 100 ml stoppered volumetric flask. It was dissolved in minimum quantity of methanol and volume was made upto 100 ml with methanol:PBS 7.4 in 3:7 and 5:5 ratio. From the above solution 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0 ml was withdrawn separately in different 10 ml volumetric flasks and volume was made up to 10 ml with 3:7 and 5:5 methanol:PBS 7.4 in each case. This produced the concentrations of 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20 μg/ml, respectively.
Absorbance of these solutions was recorded at λmax 230 nm against 3:7 and 5:5 ratio of methanol:PBS 7.4 as blank using UV/Visible spectrophotometer (Shimadzu 1800, Japan).
The linearity of an analytical method is defined as its ability to obtain test results, which are directly proportional to the analyte concentration present in the sample within a specified range. This range is depicted as the interval between upper and lower levels of analyte, which have been established to be determined with an appropriate level of linearity, precision and accuracy. For the determination of linearity, 10 concentrations ranging 2-20 μg/ml of given drug were prepared from the stock solution and least square regression analysis was employed.
Accuracy involves the determination of percentage recovery of standard compound. In this method, the calculation of percentage recovery was carried out by taking all the concentrations of drug in the pre-analyzed sample (n=3).
The intra-day and inter-day variations (repeatability) for drug determination were performed at the level of lower, medium and higher concentration over 3 levels on the same day and 3 consecutive d (Precision). The repeatability (n=6) for lower concentration only and precision (n=3) was determined and percent relative standard deviation (RSD) was calculated.
LOQ and LOD depend on the method’s sensitivity. The LOQ is the minimum sample concentration that can be measured, however LOD is the lowest concentration detected. As per International Council for Harmonisation (ICH) guidelines, there are three different methods to calculate LOD and LOQ such as visual evaluation method, signal to noise ratio method and slope method [14]. Among them, the employed method was as follows:
LOQ=10σ/S and LOD=3.3σ/S
where, σ=standard deviation of response and S=Slope of the calibration curve.
The ruggedness was studied by evaluating the similar model drug having lowest concentration by changing analyst. The change in the responses of PTX was noted in terms of percentage Relative Standard Deviation (% RSD).
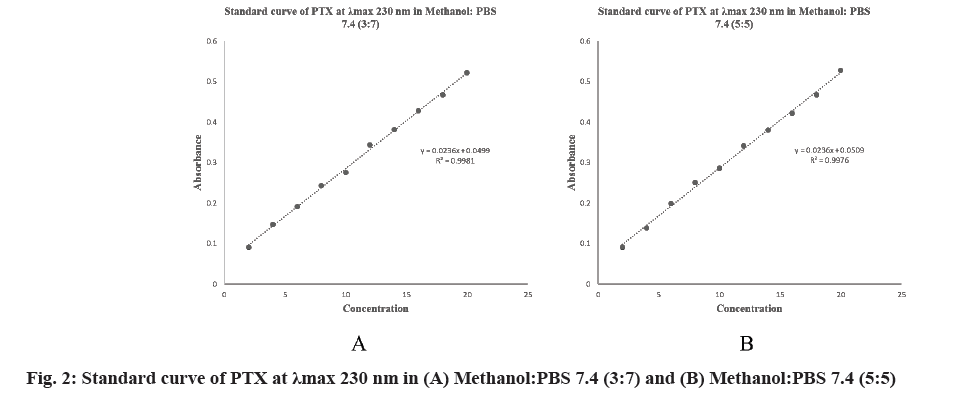
Several ratios of media methanol:PBS 7.4 were investigated for optimization of medium [15]. Finally, methanol:PBS 7.4 (3:7) and methanol:PBS 7.4 (5:5) were selected as suitable media. The spectra of PTX in both media were determined and λmax was found to be 230 nm. The linear regression equation of methanol:PBS 7.4 (3:7) was obtained at 230 nm, (0.0236×concentration in μg/ml+0.0499), with a regression coefficient of 0.9981 and (0.0236×concentration in μg/ml+0.0509) with a regression coefficient of 0.9976 for methanol:PBS 7.4 (5:5) (fig. 2).
Selected linearity range for PTX was 2-20 μg/ml and the samples was scanned at 230 nm. The linearity of the analytical for the given drug was found to be 2-20 μg/ml (r2=0.9981) in methanol:PBS 7.4 (3:7) and 2-20 μg/ml (r2=0.9976) in methanol:PBS 7.4 (5:5) and the mean slope and intercept values are within the 95 % confidence interval.
The recovery data of PTX is shown in Table 1. Results were within the acceptance criteria 98.83 %-101.63 %, indicating a good degree of sensitivity. Therefore, this method is accurate and applicable to estimate the drug. % RSD repeatability data of PTX was found to be 1.139113 (Table 2) and % RSD precision data of PTX was obtained as in Table 3. LOQ and LOD depend on the method’s sensitivity. The data were shown in Table 4. The results obtained were found to be within the specified limits. The % RSD obtained for a change of analyst was found to be less than 2. Hence the method was rugged (Table 5).
| Standard solution concentration (µg/ml) | % PTX recovered (n=3) | % RSD |
|---|---|---|
| 2 | 99.82±0.57 | 0.571028 |
| 4 | 100.77±0.86 | 0.853429 |
| 6 | 100.14±0.98 | 0.97863 |
| 8 | 99.66±0.57 | 0.571945 |
| 10 | 100.02±1.19 | 1.189762 |
| 12 | 99.82±0.65 | 0.651172 |
| 14 | 99.96±0.84 | 0.840336 |
| 16 | 100.66±0.62 | 0.615935 |
| 18 | 100.48±0.66 | 0.656847 |
| 20 | 100.42±0.48 | 0.477992 |
Table 1: Recovery Studies of PTX from Standard Samples (% PTX Recovery)
| PTX Concentration (µg/ml) | Absorbance (n=6) | % RSD |
|---|---|---|
| 2 | 0.090±0.001 | 1.139113 |
Table 2: Repeatability Data of PTX
| PTX Concentration (µg/ml) | Absorbance (n=3) | % RSD |
|---|---|---|
| Intraday study | ||
| 2 | 0.090±0.001 | 1.1111 |
| 10 | 0.286±0.004 | 1.4114 |
| 20 | 0.531±0.003 | 0.5749 |
| Interday study (2nd d) | ||
| 2 | 0.085±0.001 | 1.79 |
| 10 | 0.287±0.002 | 0.9218 |
| 20 | 0.528±0.003 | 0.3787 |
| Interday study (3rd d) | ||
| 2 | 0.088±0.001 | 1.7227 |
| 10 | 0.286±0.004 | 1.3986 |
| 20 | 0.527±0.001 | 0.3286 |
Table 3: Precision Data of PTX
| Methanol:PBS 7.4 (3:7) | Methanol:PBS 7.4 (5:5) | |
|---|---|---|
| LOD | 0.628883 | 0.715627 |
| LOQ | 1.905705 | 2.168565 |
Table 4: Limits of Detection (LOD) and Limits of Quantitation (LOQ)
| PTX Concentration (µg/ml) | Analyst-I | Analyst-II | ||
|---|---|---|---|---|
| Absorbance (n=3) | % RSD | Absorbance (n=3) | % RSD | |
| 2 | 0.090±0.001 | 1.139113 | 0.087±0.001 | 1.386 |
Table 5: Ruggedness Data of PTX
In conclusion, the above-suggested analytical technique was found to be accurate, cost-effective, simple, rapid and precise. Thus, it can be utilized for estimation of PTX in different dosage forms. The recoveries of drug from formulation were found to be decent contract with their respective claims for drug, which depicts that there is no interference of formulation excipients. Additionally, this method outnumbers the analysis time required as compared to other analytical techniques.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cancer. World Health Organization (WHO).
- Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016;176(6):816-25.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7-34.
[Crossref] [Google Scholar] [PubMed]
- Khuroo T, Verma D, Khuroo A, Ali A, Iqbal Z. Simultaneous delivery of paclitaxel and erlotinib from dual drug loaded PLGA nanoparticles: Formulation development, thorough optimization and in vitro release. J Mol Liquid 2018;257:52-68.
- Khan I, Iqbal Z, Khan A, Hassan M, Nasir F, Raza A, et al. A simple, rapid and sensitive RP-HPLC-UV method for the simultaneous determination of sorafenib and paclitaxel in plasma and pharmaceutical dosage forms: Application to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1033:261-70.
[Crossref] [Google Scholar] [PubMed]
- Andriguetti NB, Hahn RZ, Lizot LF, Raymundo S, Costa JL, da Cunha KF, et al. Analytical and clinical validation of a dried blood spot assay for the determination of paclitaxel using high-performance liquid chromatography-tandem mass spectrometry. Clin Biochem 2018;54:123-30.
[Crossref] [Google Scholar] [PubMed]
- Manning T, Plummer S, Woods R, Wylie G, Phillips D, Krajewski L. Cell line studies and analytical measurements of three paclitaxel complex variations. Bioorg Med Chem Lett 2017;27(12):2793-9.
[Crossref] [Google Scholar] [PubMed]
- Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int 2015;2015(1):413076.
[Crossref] [Google Scholar] [PubMed]
- Xie F, de Thaye E, Vermeulen A, van Bocxlaer J, Colin P. A dried blood spot assay for paclitaxel and its metabolites. J Pharm Biomed Anal 2018;148:307-15.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Zheng J. Nanoparticles for taxanes delivery in cancer treatment. J Nanosci Nanotechnol 2016;16(7):6634-47.
- Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm 2017;526(2):474-95.
[Crossref] [Google Scholar] [PubMed]
- Devi TR, Gayathri S. Estimation of paclitaxel drugs by HPLC method. Der Pharma Chemica 2010;2:109-115.
- Kesarwani P, Tekade RK, Jain N. Spectrophotometric estimation of paclitaxel. Int J Adv Pharm Sci 2011;2(2):29-32.
- Guideline IH. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005;1(20):5.
- Jadhav K, Reddy G, Chowdary Y. Determination of absorption maxima (λmax) and Beer Lambert’s range for paclitaxel by UV-visible spectrophotometer. Int J Univ Pharm Life Sci 2012;2:37-41.