- *Corresponding Author:
- Nidhi S. Patel
Department of Quality Assurance Techniques, Parul Institute of Pharmacy, Limda Ta, Waghodiya, Dist., Vadodara‑391 760, India
E‑mail: meet_krina@yahoo.com
| Date of Submission | 25 August 2013 |
| Date of Revision | 02 October 2014 |
| Date of Acceptance | 06 October 2014 |
| Indian J Pharm Sci 2014;76(6):535-540 |
Abstract
A stability-indicating reverse phase high performance liquid chromatography method was developed and validated for cefixime and linezolid. The wavelength selected for quantitation was 276 nm. The method has been validated for linearity, accuracy, precision, robustness, limit of detection and limit of quantitation. Linearity was observed in the concentration range of 2-12 μg/ml for cefixime and 6-36 μg/ml for linezolid. For RP-HPLC, the separation was achieved by Phenomenex Luna C 18 (250×4.6 mm) 5 μm column using phosphate buffer (pH 7):methanol (60:40 v/v) as mobile phase with flow rate 1 ml/min. The retention time of cefixime and linezolid were found to be 3.127 min and 11.986 min, respectively. During force degradation, drug product was exposed to hydrolysis (acid and base hydrolysis), H 2 O 2 , thermal degradation and photo degradation. The % degradation was found to be 10 to 20% for both cefixime and linezolid in the given condition. The method specifically estimates both the drugs in presence of all the degradants generated during forced degradation study. The developed methods were simple, specific and economic, which can be used for simultaneous estimation of cefixime and linezolid in tablet dosage form.
Keywords
Cefixime, Linezolid, RP‑HPLC method, forced degradation and validation
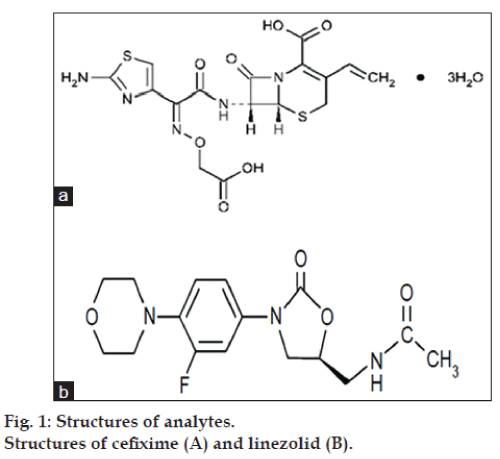
Cefixime (CEF), (6R,7R)‑7‑{ [2‑(2‑amino‑1,3‑ thiazol‑4‑yl)‑2(carboxymethoxyimino) acetyl] amino}‑3 ethenyl‑8‑oxo‑5‑thia‑1azabicyclo‑ [4.2.0] oct‑2‑ene‑2 carboxylic acid (fig. 1), is a white to light yellow, crystalline powder [1], Slightly soluble in water, soluble in methanol, sparingly soluble in anhydrous ethanol, practically insoluble in ethyl acetate [2]. It is an oral third generation cephalosporin class of antibacterial [2]. It is official in Indian Pharmacopoeia [1], British Pharmacopoeia [2] and United State of Pharmacopoeia [3], which recommends HPLC method for its analysis. Linezolid (LIN), N‑{ [(5S)‑3‑ [3‑fluoro‑4‑(mo rpholin‑4‑yl) phenyl]‑2‑oxo‑1,3‑oxazolidin‑5‑yl] methyl} acetamide (fig. 1), is a white to off‑white, crystalline powder [4], slightly soluble in ethanol, ethyl acetate and water. It is the first of the oxazolidinone class of antibiotic drug [5]. It is official in Indian Pharmacopoeia [4], which recommends HPLC method for its analysis.
CEF and LIN combination tablet is a recently introduced antibacterial combination in Indian market. Literature survey reveals that many analytical methods are reported for determination of CEF [6‑17] and LIN [18‑21] individually. Also literature survey reveals that spectrophometric methods for combined dosage form [22‑24]. However, no method is reported for simultaneous estimation of these two drugs by reverse phase HPLC.
The International Conference on Harmonization (ICH) guideline entitled “Stability testing of new drug substances and products” requires that stress testing be carried out to elucidate the inherent stability characteristics of the active substance [25]. An ideal stability‑indicating method is one that resolves the drug and its degradation products efficiently. Consequently, the implementation of an analytical methodology to determine CEF and LIN simultaneously, in presence of its degradation products is rather a challenge for pharmaceutical analyst. Therefore, it was thought necessary to study the stability of CEF and LIN under acidic, alkaline, oxidative, UV and photolytic conditions. This paper reports validated stability‑indicating HPLC method for simultaneous estimation of CEF and LIN in presence of their degradation products. The proposed method is simple, accurate, reproducible, stability‑indicating and suitable for routine determination of CEF and LIN in combined dosage form. The method was validated in compliance with ICH guidelines [26,27].
Materials and Methods
CEF and LIN of pharmaceutical grade were supplied as gift samples by West Coast Pharmaceutical Works Ltd., Ahmedabad and Alembic Pharmaceutical Pvt. Ltd., Vadodara, respectively. Potassium dihydrogen phosphate (Fischer Scientific, Mumbai), Sodium hydroxide (Rankem, New Delhi), methanol (HPLC grade, Fischer Scientific, Mumbai), nylon 66 membrane filter (0.45 μ, Himedia, Mumbai) and H2O2 (Loba Chemie Pvt. Ltd., Mumbai) were used. The Zifi Turbo tablet containing 200 mg CEF and 600 mg LIN was procured from a local pharmacy and used for analysis of marketed formulation.
The HPLC system was of LC‑20 AD (Shimadzu) with UV detector and PDA detector, a manual injection facility with 20 μl fixed loop. The chromatographic analysis was performed using LC solution software on a Phenomenex Luna C18 column (250×4.6 mm, 5 μm particle size). In addition, an electronic analytical balance (Mettler Toledo, model ML 204/A01), a pH meter (Elico, Model L1 610), a sonicator (Frontline FS 4, Mumbai, India) and a hot air oven (Biotech) were used in this study.
Preparation of mobile phase
Phosphate buffer, pH 7 was prepared by taking 50.0 ml of 0.2 M potassium dihydrogen phosphate in a 200 ml volumetric flask, to which 29.1 ml of 0.2 M sodium hydroxide was added and diluted further to the required volume with water. Six hundred millilitres of phosphate buffer pH 7 and 400 ml of methanol were mixed, sonicated for 10 min and filtered through 0.45 μm membrane filter and used as mobile phase.
Preparation of stock solutions
Stock solutions were prepared by weighing 5 mg each of CEF and LIN. The weighed drugs were transferred to two separate 50 ml volumetric flasks. Volumes were made up to the mark with mobile phase to obtain a solution containing 100 μg/ml of CEF and LIN. The HPLC analysis was performed on reversed‑phase high‑performance liquid chromatographic system with isocratic elution mode using a mobile phase of methanol: phosphate buffer pH 7 (40:60 v/v) on a Phenomenex Luna C18 column (250×4.6 mm, 5 μm particle size) with 1 ml/min flow rate at 276 nm using UV detector.
Calibration curves for CEF and LIN
Tablets contain CEF and LIN in a ratio of 1:3. Appropriate aliquots of CEF and LIN stock solutions were taken in different 10 ml volumetric flasks and diluted up to the mark with mobile phase to obtain final concentrations of 2‑12 μg/ml and 6‑36 μg/ml of CEF and LIN, respectively. The solutions were injected using a 20 μl fixed loop system and chromatograms were recorded. Calibration curves were constructed by plotting average peak areas versus concentrations and regression equations were computed for both the drugs (Table 1).
| Parameters (units) | CEF | LIN |
|---|---|---|
| Linearity range (μg/ml) | 2‑12 | 6‑36 |
| r2 | 0.9969 | 0.9983 |
| Slope | 44869 | 20282 |
| Intercept | 11856 | 21148 |
CEF: cefixime, LIN: linezolid
Table 1: Linear regression data for calibration curve
Analysis of marketed formulations
Twenty tablets were weighed, powdered, a quantity of powder equivalent to 200 mg of CEF was transferred to 100 ml volumetric flask and dissolved using mobile phase up. The solution was filtered through 0.2 μm Nylon membrane filter paper. Ten mililitres above solution was transferred to 100 ml volumetric flask and diluted up to mark with mobile phase (100 μg/ml). The sample solution was prepared to give final concentrations of 6 μg/ml and 18 μg/ml for CEF and LIN, respectively. Twenty microlitres of the above sample solution was injected into HPLC and peak areas were measured under optimized chromatographic conditions.
Method validation
The method of analysis was validated as per the recommendations of ICH [28] for the parameters like accuracy, linearity, precision, detection limit, quantitation limit and robustness. The accuracy of the method was determined by calculating percentage recovery of CEF and LIN. For both the drugs, recovery studies were carried out by applying the method to drug sample to which known amount of CEF and LIN had been added (standard addition method). At each level of the amount six determinations were performed and the results obtained were compared.
Intraday and interday precision
Intraday and interday precision study of CEF and LIN was carried out by estimating the corresponding responses 3 times on the same day and on 3 different days for the concentration of 6 and 18 μg/ml of CEF and LIN, respectively.
Limit of detection and limit of quantitation
The limit of detection (LOD) and limit of quantitation (LOQ) were calculated using following formula: LOD=3.3(SD)/S and LOQ=10(SD)/S, where SD is standard deviation of response (peak area) and S is the average of the slope of the calibration curve.
System suitability tests
System suitability tests are an integral part of chromatographic method, which are used to verify reproducibility of any chromatographic system. To ascertain its effectiveness, certain system suitability test parameters were checked by repetitively injecting the drug solution at the concentration level 6 and 18 μg/ml for CEF and LIN, respectively to check the reproducibility of the system and the results are shown in Table 2.
| Parameters (units) | CEF | LIN |
|---|---|---|
| Linearity range (μg/ml) | 2‑12 | 6‑36 |
| Correlation coefficient | 0.9969 | 0.9983 |
| LOD (μg/ml) | 0.354 | 1.886 |
| LOQ (μg/ml) | 1.074 | 5.717 |
| Recovery (%) | 98.62-101.46 | 100.04-101.12 |
| Precision (%RSD) | ||
| Interday (n=3) | 0.598-0.821 | 0.591-0.774 |
| Intraday (n=3) | 1.333-1.756 | 0.932-1.458 |
| Robustness | Robust | Robust |
| Retention time±%SD (min) | 3.213±0.015 | 11.973±0.107 |
| Resolution | ‑ | 19.482 |
| Theoretical plates | 2612 | 5443 |
| Tailing factor (asymmetry factor) | 1.073 | 0.923 |
CEF: cefixime, LIN: linezolid, LOD: limit of detection, LOQ: limit of quantitation, SD: standard deviation, RSD: relative standard deviation
Table 2: Summary of validation and sst parameters
Robustness
For robustness evaluation of HPLC method, a few parameters like flow rate and percentage of methanol in the mobile phase were deliberately changed. One factor was changed at one time to estimate the effect. Each factor selected was changed at three levels (‑1, 0, +1) with respect to optimized parameters. Robustness of the method was done at the concentration level 6 and 18 μg/ml for CEF and LIN, respectively.
Forced degradation studies
Forced degradation studies of both the drugs were carried out under conditions of hydrolysis, dry heat, oxidation and sun light. Twenty tablets were weighed, powdered, a quantity of powder equivalent to 200 mg of CEF was transferred to 100 ml volumetric flask and dissolved with mobile phase up to mark. The solution was filtered through 0.2 μm Nylon membrane filter paper. Ten millilitres above solution was transferred to 100 ml volumetric flask and diluted up to mark with mobile phase (100 μg/ml). The sample solution was prepared to give final concentrations of 6 and 18 μg/ml for CEF and LIN, respectively. This sample stock solution (100 μg/ml) was used for forced degradation studies.
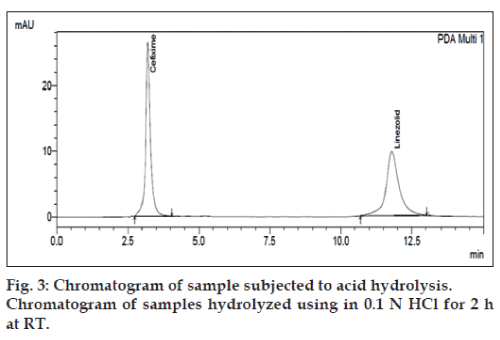
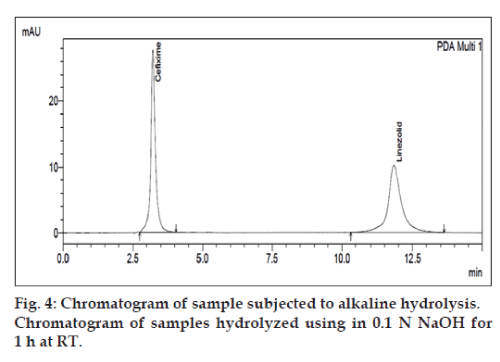
Forced degradation in alkaline condition was performed by taking 0.6 ml of sample stock solution of CEF and LIN in separate round bottom flasks. Then 0.6 ml of 0.1 N NaOH was added and this mixture was placed for 1 h at room temperature. Forced degradation in acidic condition was performed by keeping the 0.6 ml of sample stock solution in contact with 0.6 ml of 0.1N HCl for up to 2 h at room temperature. Degradation with hydrogen peroxide was performed by taking 0.6 ml of sample stock solution and adding 0.6 ml of 3% (w/v) hydrogen peroxide in the flask. This mixture was kept for up to 1 h at room temperature. For dry heat degradation, 6 μg/ml CEF and 18 μg/ml LIN solution was put in oven at 70° for 1 h. The photo stability was also studied by exposing 6 μg/ml CEF and 18 μg/ml LIN solution was put into direct sunlight at 30 min.
For HPLC analysis, all the degraded sample solutions were diluted with mobile phase to obtain final concentration of 6 μg/ml of CEF and 16 μg/ml of LIN. Similarly mixture of both drugs in a concentration of 6 μg/ml of CEF and 16 μg/ml of LIN was prepared prior to analysis by HPLC. Besides, solution containing 6 μg/ml of CEF and 16 μg/ml of LIN was also prepared without being performing the degradation of both the drugs. Then 20 μl portions of the above solutions were injected into HPLC system and analyzed under the chromatographic condition described earlier.
Results and Discussion
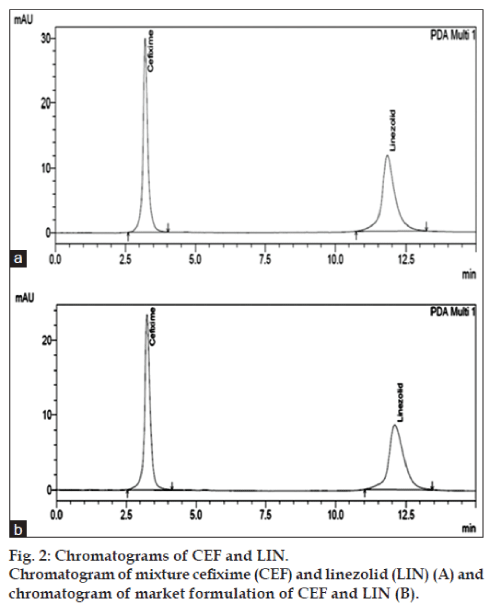
The mobile phase consisting of methanol:phosphate buffer pH 7 (40:60, v/v), at 1 ml/min flow rate was optimised which gave two sharp, well‑resolved peaks with minimum tailing factor for CEF and LIN (fig. 2). The retention times for CEF and LIN were 3.127 min and 11.986 min, respectively. UV overlain spectra of both CEF and LIN showed that both drugs absorbed appreciably at 276 nm, so this wavelength was selected as the detection wavelength. The calibration curve for CEF and LIN was found to be linear over the range of 2‑12 μg/ml and 6‑36 μg/ml, respectively. The data of regression analysis of the calibration curves is shown in Table 1. The proposed method was successfully applied to the determination of CEF and LIN in their combined tablet dosage form. The results for the combination were comparable with the corresponding labeled amounts (fig. 2).
The LOD for CEF and LIN were found to be 0.354 and 1.886 μg/ml, respectively, while LOQ were 1.074 and 5.717 μg/ml, respectively. The results for validation and system suitability test parameters are summarized in Table 2. Results for robustness evaluation for both the drugs are presented in Table 2. Insignificant differences in peak areas and less variability in retention times were observed.
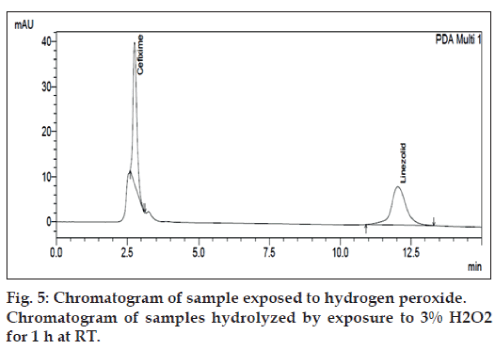
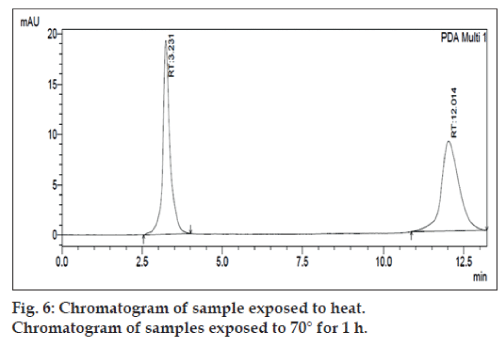
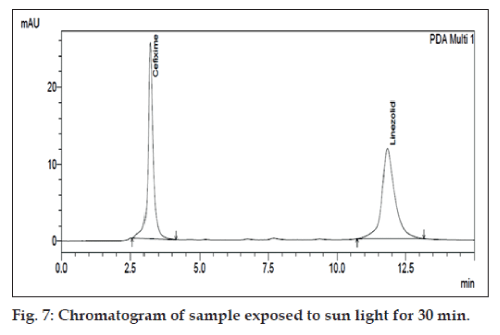
The degradation study indicated that the drug degrades as shown by the decreased areas in the peaks when compared with peak areas of the same concentration of the non degraded drug, without giving any additional degradation peaks. Percent degradation was calculated by comparing the areas of the degraded peaks in each degradation condition with the corresponding areas of the peaks of both the drugs under non degradation condition. For forced degradation with 0.1 N HCl at 2 h, 0.1 NaOH at 1 h, 3% v/v H2O2, 70° at 1 h and photo degradation at 30 min were done. The % degradation was found to be 10 to 20% for CEF and LIN in their tablet dosage form in the given condition using developed HPLC method (figs. 3‑7). Summary of degradation studies of both the drugs is given in Table 3.
| Degradation condition | Time (h) | % Degradation | |
|---|---|---|---|
| CEF | LIN | ||
| Acid (0.1N HCL) at room temperature | 2h | 5.13 | 20.52 |
| Alkali, (0.1N NaOH) at room temperature | 1h | 0.76 | 12.28 |
| Oxidation, (3% H2O2) at room temperature | 1h | 10.68 | 19.90 |
| Dry heat (70° ) | 1h | 7.52 | 13.30 |
| Direct sunlight | 0.5h | 0.48 | 16.805 |
CEF: Cefixime, LIN: Linezolid
Table 3: Summary of degradation studies for cef and lin in their tablet dosage form
In the proposed study, a stability‑indicating HPLC method was developed for the simultaneous estimation of CEF and LIN and validated as per ICH guidelines. Statistical analysis proved that method was accurate, precise, and repeatable. The developed method was found to be simple, sensitive and selective for analysis of CEF and LIN in combination without any interference from the excipients. The method specifically estimates both the drugs in presence of all the degradants generated during forced degradation study. Assay results for combined dosage form using proposed method showed 99.19±0.454% of CEF and 98.93±0.757% of LIN. The results indicated the suitability of the method to study stability of CEF and LIN under various forced degradation conditions acid, base, dry heat, oxidation and photolytic degradation. It can be concluded that the method separates the drugs from their degradation products; it may be employed for analysis of stability for their tablet dosage form. However, characterization of degradation products was not carried out.
Acknowledgements
The authors express their gratitude to West Coast Pharmaceuticals Works Ltd., Ahmedabad, Gujarat and Alembic Pharmaceuticals Pvt. Ltd., Vadodara, Gujarat for the gift sample of pure CEF and LIN. They also thank Dr. K. S. Rajesh, Principal, Parul institute of Pharmacy and Dr. Arvind Badiger, Technical Director, BDR Pharmaceuticals International Pvt. Ltd., Vadodara for providing necessary facilities and his constant support.
References
- Indian Pharmacopoiea. Vol. 2. New Delhi: Controller of Publication, Govt. of India, Ministry of Health and Family Welfare; 2010. p. 1012.
- British Pharmacopoiea, Vol. 1. London: HMSO Publication; 2009. 397. P: 397.
- United State of Pharmacopoeia 31 National Formulary 26, Vol. 2. The standard of quality. United State Pharmacopoeial Convention Inc. 2009. 1671.
- Indian Pharmacopoiea. Vol. 2. New Delhi: Controller of Publication, Govt. of India, Ministry of Health and Family Welfare; 2010. p. 1590.
- Rang HP, Dale MM, Ritter JN, Moore PK. Pharmacology: 6th ed. New York: Churchill Livingstone; 2003. p. 668.
- Khaja P, Patil CS, Vijaykumar K, Ali S, Chimkod VM. Reverse phase HPLC method for the determination of cefixime in pharmaceutical dosage forms. Res J Pharm Bio Chem Sci 2010;1:226‑30.
- Dragica Z, Traje S, Petar M. High‑performance liquid chromatographic method for determination of cefixime and cefotaxime in human plasma.Bulletin Chemists Techno Macedonia 2003;22:39‑45.
- Azhagesh RK, Divya Y, Deepthi Y, Prabu C, Manikantan S. Determination of cefixime trihydrate and cefuroxime axetil in bulk drug and pharmaceutical dosage forms by HPLC. Int J Chem Tech Res 2010;2:334‑6.
- Kathiresan K, Murugan R, Shahul HM, Gokula IK, Taranath K. Analytical method development and validation of cefixime and dicloxacillin tablets by RP‑HPLC. Rasayan J Chem 2009;2:588‑92.
- Kapil SK, Santosh VG, Padmanbh BD, Nilesh VG. A simple and sensitive RP–HPLC method for simultaneous estimation of cefixime and ofloxacin in combined tablet dosage form. Int J Pharm Pharm Sci 2011;3:46‑8.
- Khan IU, Sharif S, Ashfaq M, Asghar MN. Simultaneous determination of potassium clavulanate and cefixime in synthetic mixtures by high‑performance liquid chromatography. Asian J Anal Chem 2008;91:744‑9.
- Sudhakar M, Venkateshwara RJ, Devika GS, Ramesh PR. A validated RP‑HPLC method for simultaneous estimation of cefixime trihydrate and ornidazole in tablet dosage forms. Int J Chem Pharm Sci 2010;1:34‑9.
- Shah C, Umalkar D, Rajesh KS. Development of an RP HPLC and force degradation of cefixime and moxifloxacin in bulk and pharmaceutical dosage form. Int J Pharm Res Bio Sci 2012;1:128‑47.
- Dhoka MV, Gawande VT, Joshi PP. Simultaneous estimation of cefixime trihydrate and erdosteine in pharmaceutical dosage form by using reverse phase – high performance liquid chromatography. Int J Chem Tech Res 2010;2:79‑87.
- Gandhi SP, Rajput SJ. Study of degradation profile and development of stability indicating methods for cefixime trihydrate. Indian J Pharm Sci 2009;71:438‑42.
- Sutar SV, Warjurkar RP, Pishwikar SA. Spectrophotometric method for degradation study of cefixime trihydrate. Asian J Biomed Pharm Sci 2012;2:50‑4.
- Elsadig HK, Ahmed EM, Izzeldin EB. Study of degradation of cefixime trihydrate under stress conditions using stability indicating reverse phase‑high performance liquid chromatography method. Der Pharm Chemica 2011;3:197‑207.
- Jaya PK, Syama SB. A validated RP‑HPLC method for the determination of linezolid in pharmaceutical dosage forms. Int J Pharm Bio Sci 2012;3:44‑51.
- Abdel KM, Weshahy SA, Dina SS. Validated stability indicating assay of linezolid by spectrophotometric and high performance liquid chromatographic methods. Aust J Basic Appl Sci 2012;6:767‑78.
- Agrawal H, Mahadik KR, Paradkar AR, Kaul N. Stability indicating HPTLC determination of linezolid as bulk drug and in pharmaceutical dosage form. Drug Dev Ind Pharm 2003;29:1119‑26.
- Khasia VD, Khasia HV, Desai D, Bhakhar DN, Parmar AR. Development and validation of stability indicating RP‑HPLC method for linezolid immediate release tablet dosage form. J Pharm Res 2012;5:4115‑8.
- Helly S, Payal P, Khushbu P, Sagar S. Method development and validation of spectrophotometric methods for simultaneous estimation of cefixime trihydrate and Linezolid in their combined tablet dosage form. Int J Pharm Res Bio Sci 2012;1:516‑29.
- Patel DP, Goswami K, Patel M. Development and validation of UV spectrophotometric method for simultaneous estimation of cefixime and linezolid in combined dosage. Int J Pharm Res Scholars 2012;1:112‑8.
- Thakkar D, Mashru R. Simultaneous estimation of cefixime trihydrate and linezolid in combine pharmaceutical formulation. Int J Pharm Pharm Sci 2013;5:76‑81.
- ICH, Q1A, Stability Testing of New Drug Substances and Products. In: Proceedings of the International Conference on Harmonisation, Geneva, Oct 1993.
- ICH, Q2A, Hamonised Tripartite Guideline, Test On Validation of Analytical Procedures, IFPMA. In: Proceedings of the International Conference on Harmonization, Geneva, Mar 1994.
- ICH, Q2B, Hamonised Tripartite Guideline, Validation of Analytical Procedure: Methodology, IFPMA, in: Proceedings of the International Conference on Harmonization, Geneva, Mar 1996.
- ICH Guidance on Analytical Method Validation, in: Proceedings of the International Convention on Quality for the Pharmaceutical Industry, Toronto, Canada, and September, 2002.